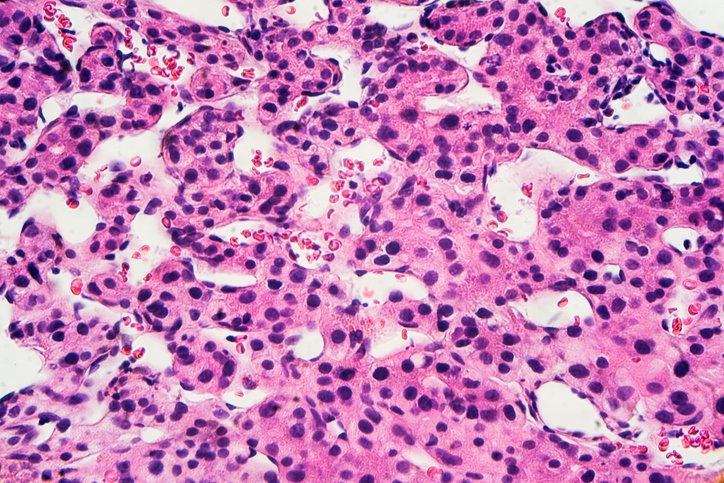
What if cutting off a tumor’s fat supply could also rally the immune system to join the fight? Liver cancer fueled by fatty liver disease is on the rise, and with low survival rates, new treatment strategies are urgently needed. A new study suggests that blocking fat metabolism does just that—turning immune cold tumors into immune hot zones.
In a study published recently in Nature titled “ACLY inhibition promotes tumor immunity and suppresses liver cancer,” the team reported that inhibiting ATP citrate lyase (ACLY)—a key enzyme that converts glucose into fat—reprograms the tumor microenvironment and triggers a potent immune response. Surprisingly, that response is led not by the usual cancer-fighting T cells, but by B cells.
“This study demonstrates that targeting tumor metabolism, specifically via ACLY inhibition, can reprogram the tumor microenvironment to enhance immune surveillance,” senior author Gregory Steinberg, PhD, told GEN. “By genetically or pharmacologically suppressing ACLY, we not only reduced tumor proliferation and intratumoral fat accumulation, but also stimulated immune infiltration, notably and surprisingly B cells, which were essential for tumor clearance.
“Tumors rely on altered metabolism to sustain growth and evade immune detection. In MASH-HCC [metabolic dysfunction–associated steatohepatitis-driven hepatocellular carcinoma], this metabolic rewiring is compounded by the liver’s unique exposure to nutrients, cytokines, and microbial metabolites, creating an immunosuppressive niche. We saw an opportunity to disrupt this through ACLY,” added Steinberg.
The work centers on a novel small molecule called EVT0185 (6-[4-(5-carboxy-5-methyl-hexyl)-phenyl]−2,2-dimethylhexanoic acid), which was identified via phenotypic screening. Unlike other ACLY inhibitors, EVT0185 is selectively activated in liver and tumor cells—sparing immune cells (a feature essential for combination with checkpoint therapies, Steinberg noted)—and was effective across multiple mouse models of MASH-HCC. These cancers, often arising in patients with advanced fatty liver disease, are notoriously resistant to current immunotherapies.
“When we started this project, we did not expect to see an immune-driven phenotype on tumors with ACLY inhibition,” said Steinberg. “However, we consistently observed dramatic increases in tumor-infiltrating B cells and formation of tertiary lymphoid structures (TLSs)…Perhaps most striking was that depleting B cells eliminated the therapeutic benefit of ACLY inhibition, highlighting their central role.”
Beyond tumor shrinkage, the study revealed that EVT0185 increases levels of CXCL13—a chemokine that attracts B cells—and supports the formation of TLSs, which are linked to improved outcomes in HCC. The effects were observed using a genetic model, spatial and single-cell transcriptomics, and single-cell proteomics.
Tumor metabolism has long been considered a hallmark of cancer, but its role in immune evasion is gaining new attention. “Unlike many approaches that target surface receptors or immune checkpoints, we’re going upstream, shifting the metabolic programming of tumor cells to make them more visible to the immune system,” said Steinberg. “This is especially important in MASH-HCC, where conventional immunotherapies show limited efficacy.”
When asked what inspired this research, Steinberg told GEN, “My background is in studying obesity and type II diabetes, where not only in adults but also in children, we’ve seen a dramatic rise in early-onset metabolic disease over the last two decades. That experience led me, about a decade ago, to start thinking seriously about the emerging epidemic of MASH-HCC. The liver is uniquely vulnerable in the context of chronic metabolic dysfunction, and it was clear that we were on the brink of seeing steatohepatitis-driven cancers become a major clinical challenge.
What concerned me most is that MASH-HCC often arises silently, without cirrhosis, and these tumors tend to be resistant to current immunotherapies due to their immunosuppressive microenvironment. This disconnect—between the rising burden of metabolic liver cancer and the poor response to existing treatments—is what catalyzed our efforts to develop a targeted, tumor-specific metabolic therapy.”
The researchers are now preparing EVT0185 for clinical trials. “We’ve completed preclinical efficacy and safety studies… It’s now ready for clinical translation,” shared Steinberg.
More broadly, the findings redefine how researchers view immune reprogramming in cancer. “B cells are often overlooked in the immuno-oncology space,” said Steinberg. “But our data… consistently showed a robust B cell–driven immune response. We believe this reshapes how we think about immune reprogramming in cancer.”
By shifting away from conventional approaches, such as targeting oncogenic signaling or checkpoint pathways, the researchers have “shown that metabolic rewiring can act as an upstream immunotherapeutic lever. Importantly, these effects extend across multiple models and link directly to clinical observations in human MASH-HCC samples, laying a foundation for a new class of immunometabolic therapies.”
The post B Cell–Driven Immunometabolic Strategy Halts Liver Tumors in Mice appeared first on GEN – Genetic Engineering and Biotechnology News.