Introduction
Vascular endothelial growth factor A (VEGF-A, also known as VEGF) dependent activation of VEGFR2 is the main driver of angiogenesis by stimulating proliferation and migration of endothelial cells and regulating cell-adhesion and vascular permeability processes1. VEGF upregulation is implicated in pathologies involving mis regulation of ocular angiogenesis, and it is implicated in various diseases including proliferative diabetic retinopathy, retinopathy of prematurity, corneal neovascularization, retinal vein occlusion and age-related macular degeneration (AMD)2. AMD is the leading cause of blindness for individuals above 55 years of age if left untreated3. AMD presents with fatty protein deposits in the retinal pigmented epithelium (RPE) at early stages, whereas late-stage AMD can present with neovascularization originating from the choroid and extending into the macular region of the retina. This neovascular form is also known as wet AMD due to excessive permeability (leakiness) of the abnormally formed choroidal vessels4,5. VEGF expressed in RPE regulates choriocapillaris and it is highly upregulated in wet AMD. VEGF is a key target in therapeutic intervention of wet AMD and potentially other diseases involving pathological neovascularization of the retina, since it is a critical regulator of both angiogenesis and vascular permeability6. VEGF drives the development of choroidal neovascularization where new vessels grow through RPE. VEGF accumulation in wet AMD can be treated by administration of anti-VEGF therapeutics into the eye. Ranibizumab is the first anti-VEGF antibody fragment developed and approved for use in wet AMD treatment7. Later, its use in treatment of diabetic retinopathy and diabetic macular edema was also approved8. Aflibercept (Eylea) is a chimeric VEGFR1-VEGFR2 fusion protein that also neutralizes VEGFA, proven to be effective in AMD treatment9. More recently, brolucizumab (Beovu) has become the first single chain variable fragment (scFv) anti-VEGF to be approved for clinical use10,11. However, immunogenicity cases of intraocular inflammation in brolucizumab treated patients raised some concerns12,13. Interestingly, anti-drug antibodies were detected in patients treated with brolucizumab while ranibizumab did not have a similar effect14.
scFv is a single chain antibody fragment containing variable light and heavy chains of an antibody linked by a flexible peptide linker. This linker is usually 15–20 amino acids long and made up of glycine and serine residues for increased solubility and flexibility15. scFvs usually have strong binding affinities to their antigens, and better diffusion in tissues due to their smaller size (~ 27 kDa) compared to full-length IgG (150 kDa)16. Furthermore, scFvs can be easily produced in microbial systems, rather than more expensive mammalian systems which are usually required for full-length antibodies15.
In this study, we generated novel anti-VEGF scFv antibodies derived from ranibizumab. Compared to ranibizumab, two of those scFv variants showed key technical advantages such as more potent anti-mitogenic and anti-angiogenic activity in human cell-based systems and in in vivo zebrafish animal models. One lead scFv was selected for its superior activity in inhibiting VEGF driven HUVEC proliferation, VEGFR2 activation, in vivo angiogenesis and vessel permeability. Finally, this lead molecule was shown to inhibit tubulogenesis better than ranibizumab. The novel superior scFv1 derived from ranibizumab sequence, performs similar to brolucizumab, but it is much more effective than the ranibizumab in regulation of biological processes driven by VEGF. Overall, this study shows that smaller antibodies derived/engineered from a successful template can have superior bioactivity.
Materials and methods
Cloning and protein expression
Anti-VEGF scFv design was based on ranibizumab sequence obtained from the DrugBank database with the accession number DB0127017. Glycine-serine linker of (G3S)5 was used and antibody residues were numbered according to the Kabat numbering system. The initial sequence is annotated as scFvWT and more than 30 mutations were generated. Genes were codon optimized for Pichia pastoris (P. pastoris) and obtained from GenScript. The genes were ligated into the XhoI/NotI restriction sites of the P. pastoris expression vector pPICZαA (V195-20, Thermo Fisher Scientific, Waltham, MA, USA). Approximately 1 µg of the expression plasmid linearized with PmeI (New England Biolabs, Ipswich, MA, USA) was transformed into P. pastoris X33 host cells as described before18. The plasmid was targeted to the AOX1 locus. Transformed cells were cultivated and transformants were selected in YPD (1% yeast extract, 2% peptone, 2% dextrose) agar with 100 µg/mL zeocin after incubation for 2–3 days at 30 °C. Gene copy number was determined with qPCR as described before19. Colonies were selected and corresponding research cell banks were produced as described in our previous study18,20.
Shake-flask production
The selected clones were grown in 3 mL YPD broth and inoculated to BMGY media (1% yeast extract, 2% soytone, 1 M potassium phosphate buffer pH 6.0, 13.4 g/L Yeast Nitrogen Base, 10% Glycerol, 4 × 10−5 g/L Biotin) overnight at 28 °C and 225 rpm in a shaking incubator. The cells were grown overnight, centrifuged, and transferred to a BMMY media (1% yeast extract, 2% soytone, 1 M potassium phosphate buffer pH 6.0, 13.4 g/L yeast nitrogen base, 10% methanol, 4 × 10−5 g/L biotin) to induce protein induction with methanol. During the 96-h production period, 1% of the final methanol concentration was added to the culture medium every 12 h. The supernatant samples were taken at regular intervals for SDS-PAGE analysis.
Protein purification
Proteins were purified from culture supernatants with ÄKTA Avant FPLC system. Supernatant pH was adjusted to 7.0–7.2 range before loading it onto the Protein L HiTrap (MabSelect VL, Cytiva, Wilmington, DE, USA) column. The column was equilibrated and washed with 20 mM Na2HO4P, 150 mM NaCl, pH 7.1 ± 0.1 or 4.5 mM acetic acid, 45.4 mM sodium acetate, 500 mM NaCl pH 5.7 ± 0.1 wash buffer. Culture supernatant was loaded onto the column at 155.9 cm/h, column was washed with 4 column volumes of the wash buffer. Proteins were eluted with linear gradient in 4.01 mM Na2HO4P, 89 mM citric acid pH 2.2 and pH was neutralized with 1 N Tris-HCl pH 8.0 buffer at the bottom of elution pools. Eluates were pooled and concentrated using 10 K MWCO ultra-centrifugal filters (UFC9010, Merck, Darmstadt, Germany). Proteins were further purified, and were buffer exchanged by Size Exclusion Chromatography (SEC) with HiLoad 16/600, Superdex 75 pg pre-packed column (28989333, Cytiva, Wilmington, DE, USA) with 7.13 mM NaH2O4P, − 12.87 mM Na2HO4P, 150 mM NaCl pH 7.2 at 29.8 cm/h. The monomer peak was collected as an elution. The final purified pool was analyzed with SE-UPLC for product-related impurities. Pooled protein was concentrated to ~ 10 mg/mL final concentration with 10 K MWCO ultra-centrifugal filters for further use.
Thermal denaturation assay
Thermal unfolding profiles of purified scFv proteins were determined with SYPRO Orange dye-based RT-PCR (S5692, Sigma Aldrich, Darmstadt, Germany), and thermogram data was calculated with the Hill1 equation fit using the Origin 8.5 software according to published protocols15.
Surface plasmon resonance (SPR)
VEGF affinity of scFv variants was measured with a Biacore T200 instrument (Biacore Inc., Piscataway, NJ) with human VEGF coated CM5 chip as described previously15. Sensorgram curves were analyzed using the BiaEval 3.0 manufacturer’s software. Sensorgrams were reference-subtracted and analyzed using Biacore T200 Evaluation Software. Global fitting was performed using a 1:1 Langmuir binding model to derive kinetic parameters. The dissociation constant (KD), association rate constant (kon) and dissociation rate constant (koff) values were calculated by fitting the kinetic association and dissociation curves to a 1:1 binding model. VEGF family/isoform/ortholog affinities were analyzed with a scFv1/ranibizumab/brolucizumab coated CM5 chip. The same concentration of VEGFs was injected over immobilized chips, and the binding signals (RU) were normalized among each other.
VEGF bioassay
VEGF bioassay (GA2005, Promega, Madison, WI, USA) was used to measure inhibitory doses of scFv variants. The test was performed according to the product manual. Briefly, 40,000 KDR/NFAT-RE HEK293 cells were seeded per well. VEGF165 (J2371, Promega, Madison, WI, USA) EC50 was determined to be 7.3 ng/mL, with Hill Slope of 0.5595. EC80 concentration was calculated to be 88 ng/mL with Hill Slope (H) equation:
ECF = (F/(100 – F))1/H. EC50
Bevacizumab (Altuzan, Roche, Basel Switzerland), ranibizumab (Lucentis, Novartis, Basel, Switzerland), scFvWT and scFv1/2/3 variants were titrated between 6 and 0.000914 µg/mL with 3-fold dilution to obtain dose response curves. Luciferase signal was recorded with a luminometer (Centro LB360, Berthold Technologies, Germany) in triplicate. IC50 concentrations were calculated after Sigmoidal, 4PL non-linear regression fitting of the log(concentration) vs. normalized luciferase values (OriginPro version 2024, OriginLab Corporation, Northampton, MA, USA).
Proliferation inhibition test
HUVEC cells (C2519AS, Lonza, Basel, Switzerland) were seeded 10,000 cells per well. MTT (M5655, Sigma-Aldrich, St Louis, MI, USA) cytotoxicity test was performed according to ISO 10993-5, ICH guidelines. Briefly, recombinant VEGF165 (J2371, Promega, Madison, WI, USA) was applied at 4 nM, ranibizumab, scFvWT and 3 variants were titrated between 0.125 nM − 6 nM doses. Absorbance at 570 nm was recorded with a microplate reader (BioTek Synergy H1, Agilent Technologies, Santa Clara, CA, USA). IC50 were calculated after 4PL fitting of log(molarity) vs. the absorbance values, using GraphPad Prism (version 8.0.1, GraphPad Software, Boston, Massachusetts USA).
Zebrafish angiogenesis inhibition test
Embryos of transgenic fli1:EGFP line crossed to wild type AB zebrafish were incubated in embryo medium at 28 °C in a dark incubator21. Subintestinal vein (SIV) inhibition was tested via injection of anti-VEGFs to the yolk sac of 2 days post fertilization (dpf) zebrafish as described previously15. 49 nL of 55 µM antibody fragment suspensions were injected to each embryo.
Retinal vessel leakage assay
Zebrafish embryos were co-treated with 5 µg/mL anti-VEGF antibodies with 5 mM CoCl2, between 1 and 5 dpf. Dextran, Tetramethylrhodamine (TAMRA), 2,000,000 MW (D7139, Thermo Fisher Scientific, Waltham, MA, USA) was injected into larval vasculature at 4 dpf. The larvae at 5 dpf were embedded in 1% low melting agarose and imaged live with Zeiss LSM 880 confocal microscope. To measure the TAMRA signal, signal in 8 fixed size regions of interest (roi) around the retinal vessels were measured, for each larva. Signal was normalized by subtracting the general background signal recorded from an area outside of the vascularized area on the images. 5 independent larvae were used for each condition and average TAMRA leak was displayed on graph.
Angiogenesis assay
Geltrex™ (A14132-02, Gibco, NY, USA) was thawed overnight at 4 °C on ice due to rapid gelation at 22 °C to 35 °C. µ-Slide 15 Well 3D (81506, Ibidi, Gräfelfing, Germany) wells were filled with 10 µl Geltrex™ and kept on ice. µ-Slide was sealed with the supplied lid and incubated at 37 °C and 5% CO2 for 30 min. 10,0000 HUVEC cells were seeded per well. Briefly, Recombinant VEGF165 was applied at 1 nM, ranibizumab and scFv1/2 was applied at 2 nM. 50 µL of cell suspension and prepared treatment groups were added to each well. The angiogenesis assay plate µ-Slide was incubated at 37 °C in a 5% CO2 atmosphere for 10 h. Calcein AM (354216, Corning, Bedford, MA, USA) solution was prepared at 8 µg/mL. 1 µl was added to each well. Incubation was performed for 30 min at room temperature. Apotome fluorescence microscope (Zeiss Observer 7 with Apotome 2.0, Oberkochen, Germany) was used for imaging. Angiogenesis analyzer software was added to Image J and analyzed. Total length, total branching length, total segment length and total branch length were calculated by 2-way ANOVA followed by Dunett’s multiple comparison test22.
Results
Anti-VEGF single chain variable fragment (scFv) variants with improved VEGF affinities were produced
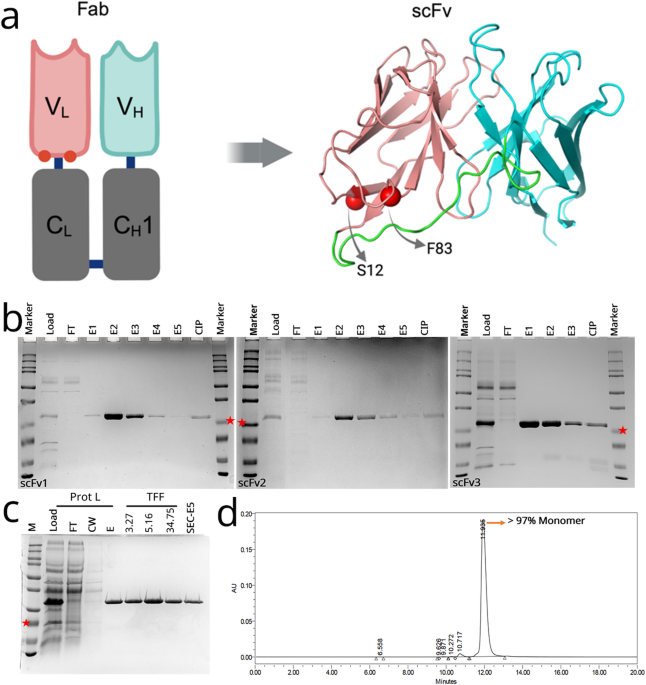
An scFvWT was designed based on the sequence of ranibizumab, and more than 30 novel anti-VEGF sequences were generated with rational mutagenesis. After initial screening for production, solubility and stability, three lead scFv variants (scFv1: F83E, scFv2: S12M, scFv3: F83E-S12M) with mutations in the variable light chain were selected for further studies. Light chain residues of F83 and S12 are both located at the interface of Fab VL-CL which is absent in our scFv design (Fig. 1A). F83 is a critical residue associated with the dynamics of Fab elbow angle, and the interaction between VL and CL through its hydrophobic core interactions23. Since F83 is exposed in the scFv design, it was mutated to a charged residue of glutamate to increase solubility and stability. S12 was chosen due to its critical H-bond interaction with CL interface23, mutation to methionine was chosen to keep VL domain more compact.
Structure, production, and purification of anti-VEGF scFv variants. (A) Graphic representing the location of mutated residues (red spheres) in the original ranibizumab template, and the modelled structure of the scFv variant. Positions for selected mutations of F83E and S12M are represented by red spheres. Variable heavy chain (VH), light chain (VL) and linker are colored cyan, salmon and green, respectively. (B) Protein L-purified proteins were analyzed on SDS-PAGE gel for scFv1 (left), scFv2 (middle), scFv3 (right). FT: flow-through, E1-E5: Elution fractions, CIP: Clean-in-place (proteins stuck onto the Protein L column). scFv bands at around 25 kDa (indicated with red stars). (C) Samples from Protein L purification and protein concentration steps are analyzed on SDS-PAGE. Prot L: Protein L Chromatography, TFF: Tangential Flow Filtration, FT: Flow-Through, CW: Column Wash, E: Elution, SEC: Size Exclusion Chromatography. (D) Representative SEC-HPLC chromatogram of the purified scFv1. Detection at 280 nm absorbance (mAU) and conductivity (mS/cm) was plotted. Monomer peak was observed at 12 min.
Variants were transformed and produced in P. pastoris. Clones were screened for production and selected colonies were found to have around 12 copy number of the inserted scFvs (data not shown). A shake flask production and purification were conducted for each variant and proteins were analyzed in SDS-PAGE (Fig. 1, Figure S 1). Protein L capture eluted proteins with > 85% purity (Fig. 1A). Buffer exchange and protein concentration studies were conducted with tangential flow filtration (TFF) before secondary purification of size exclusion chromatography (SEC) (Fig. 1B). SEC yielded > 97% purity, representative plot of scFv1 is shown in (Fig. 1C). Next, protein stabilities were assessed with a thermal denaturation assay using the Sypro Orange Dye. Tm values were found to be 54.5 ± 0.16 °C, 56.7 ± 0.22 °C, 55.7 ± 0.17 °C and 53.9 ± 0.13 °C for scFvWT, scFv1, scFv2 and scFv3, respectively (Table 1). scFv1 and scFv2 had a slightly better stability than the scFvWT. Affinities of these scFv variants for the human VEGF protein were tested with surface plasmon resonance. scFv1 displayed slightly improved affinity to the VEGF when compared to scFvWT while scFv2 had the highest affinity of 24.4 pM (Table 1; Fig. 2).
SPR binding kinetics and melting temperatures of the anti-VEGF scFvs. SPR sensorgrams along with used antibody concentrations of (A) scFWT, (B) scFv1, (C) scFv2, (D) scFv3.
Biological activities of the variants
Biological activity of the three variants was assessed with a 3-(4, 5-dimethylthiazolyl-2)−2, 5-diphenyltetrazolium bromide (MTT) test to determine IC50 values of each variant needed for inhibition of VEGF induced proliferation of human umbilical vein cells (HUVECs). 4 nM (100 ng/mL) VEGFA was applied to induce proliferation, and ranibizumab, scFvWT and scFv1/2/3 were titrated between 0.0625 nM − 6 nM doses. IC50 of ranibizumab was found to be 4.42 nM. While IC50 values scFv2 (4.47 nM) and scFv3 (3.97 nM) were close to that of ranibizumab, scFv1 had the best IC50 value of 2.39 nM (Fig. 3A).
Inhibitory activity of the scFv variants. A) Inhibition of VEGF induced HUVEC proliferation was tested with an MTT assay. Three variants were compared to the reference ranibizumab. B) VEGF induced VEGFR2 activity test with a VEGF bioassay kit was analyzed with normalized luminescence values for 9 different concentrations of anti-VEGFs. scFv1/2/3 variants and scFvWT were compared to bevacizumab, ranibizumab, brolucizumab. Bevacizumab (red), ranibizumab (green), brolucizumab (navy), scFvWT (gray), scFv1 (orange), scFv2 (blue), scFv3 (purple). IC50 was calculated after 4PL fitting of log(molarity) vs. the absorbance values, using GraphPad Prism (version 8.0.1, GraphPad Software, Boston, Massachusetts USA).
VEGF Bioassay is an assay that measures VEGF induced VEGFR2 activation in a luminescence based test validated by ICH guidelines24. For a sensitive comparison of biological activities, activities of scFvWT and three variants scFv1/2/3, commercially available reference antibodies bevacizumab, ranibizumab and brolucizumab were directly compared (Fig. 3B). IC50 values of bevacizumab (0.6185 µg/mL) and ranibizumab (0.2429 µg/mL) were found to be similar to the concentrations reported in the validated assay manual. The scFvWT performed the worst with IC50 of 2.102 µg/mL. The double mutant, scFv3, had an IC50 value of 0.2685 µg/mL and displayed a plot comparable to that of ranibizumab. On the other hand, both scFv1 (0.1215 µg/mL) and scFv2 (0.1029 µg/mL) outperformed bevacizumab and ranibizumab (Fig. 3B). scFv1 and scFv2 plots were similar to brolucizumab which had an IC50 value of 0.1045 µg/mL.
According to the VEGF Bioassay, scFv1 and scFv2 performed better than scFv3 and ranibizumab. However, in the proliferation inhibition assay, scFv1 performed the best while scFv2 and scFv3 were similar to ranibizumab. Therefore, we decided to compare scFv1 and scFv2 in an alternative model and used the zebrafish subintestinal vein (SIV) inhibition assay15. To this end, anti-VEGFs were injected into the yolk sac of the 2 days post fertilization (dpf) embryos to neutralize the endogenous VEGF that induces formation of SIVs from the dorsal vein (Fig. 4A). Embryos were imaged at 3 dpf and the area of the SIV was measured and quantified in each embryo. When SIV was imaged with confocal microscopy, inhibition of angiogenesis was observed in scFv1 or scFv2 injected embryos (Fig. 4B). In ranibizumab or scFvWT injected embryos SIV formation was similar to that of control group (PBS) at 3 dpf (Fig. 4C). Average SIV area analysis showed scFv1 had the best performance by reducing the SIV area by half when compared to ranibizumab or scFvWT injected groups, whereas scFv2 was less efficient (Fig. 4C). While some of the embryos injected with scFv1 or scFv2 had morphological defects, morphologies of embryos were normal in PBS control, ranibizumab, and scFvWT injected groups. The observed toxicity was analyzed by analysis of embryo morphologies, and as shown in Fig. 4D scFv2 caused more toxicity leading to the death of ~ 30% of the embryos. Finally, the efficiency of the anti-VEGFs was tested in a zebrafish embryonic leaky retina model that mimics the wet AMD phenotype. To this end, zebrafish larvae were kept in chemically induced hypoxic conditions, which causes leaky retinal vessels as indicated by leakage of the high molecular weight fluorescently conjugated dextran (Fig. 5A, B)25. The red dye was injected into the vasculature and retinal vessels were imaged with the confocal microscope. The CoCl2 induced hypoxic condition caused leakage of the red dye from the vessels, ranibizumab and scFv2 treatment did not prevent leakage, whereas scFv1 was the most effective in preventing the leak (Fig. 5B – G).
In vivo angiogenesis inhibition tests. Anti-VEGFs were injected into the yolk of 2 dpf embryos, prior to the onset of SIV angiogenesis and embryos were imaged with stereomicroscope at 3 dpf. (A) Overview images of the transgenic fish showing vasculature (left), representative close-up images of SIV recorded at the confocal microscope (right). (B) Representative images showing the morphologies of injected embryos at 3 dpf. Whole body images were acquired with brightfield (right) and fluorescent stereomicroscope (left) and SIV images were acquired with confocal microscope (middle). Statistical significance was tested with one-tail T-test with a confidence interval of 95%, with Excel software (Microsoft, Redmond, WA, USA). Error bars represent SD. ns: non-significant, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001. (C) Plot of average SIV area of each group is presented. SIV area was measured in 24 embryos (normal or defective morphology) in each experimental group. Two independent experiments were conducted with similar results. (D) Plot showing ratio of healthy (blue), defective (orange) or dead (black) embryos injected with antibodies or PBS. Sample size (n) = 40 (PBS), 38 (ranibizumab), 37 (scFvWT), 36 (scFv1), 39 (scFv2) 39. Two independent experiments were conducted with similar results.
Inhibition of retinal leakage. (A) Graphic representation of the experimental design. Zebrafish embryos were kept in embryo medium containing CoCl2 and antibodies to be tested from 1–5 dpf. TAMRA dextran was injected to the vasculature at 4 dpf larvae and retinal vessels were imaged at 5 dpf. (B) Dye intensity was measured in every retina, in different comparable positions. The number of ROIs and locations are indicated on the image with circles. Larval background signal (green circles), signal in the vein (red circles) and signal around the vessels (yellow circles). Plot shows normalized average leak intensity in each group (n = 5) larvae per group, 2 independent experiments were conducted, similar results were obtained. Results of one experiment is displayed. Statistical significance was tested by one-way ANOVA followed by Kruskal-Wallis comparison test. Error bars represent ± SD ns: non-significant, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001. C – G) Representative images from each experimental group. Overlay image (first row), TAMRA image (second row) is shown for each condition. (C) Control larva that was maintained in normal embryo medium, (D) negative control larva that was treated with CoCl2 but did not receive any anti-VEGF, (E) ranibizumab treated, (F) scFv1 treated and (G) scFv2 treated larva.
Lead variant scFv1 prevents angiogenesis and binds to VEGFA selectively
Based on the results reported above scFv1 was selected as the lead variant. To confidently propose the scFv1 as a therapeutic molecule candidate, it was further tested with a HUVEC in vitro angiogenesis assay26 (Fig. 6). 2 nM VEGF induced tubular network organization of HUVECs seeded in a matrix within 4 h (Figs. 6A, B). When 2 nM ranibizumab or scFv1 was added, the tubular network formation was inhibited (Figs. 6C, D). The length of tubes, branches and length of segments were quantified with a dedicated ImageJ tool22. Quantification of three independent wells was performed, and averages were plotted (Fig. 6E). All tested parameters demonstrated superiority of scFv1 over ranibizumab in this angiogenesis assay. Finally, VEGF selectivity/specificity and species cross reactivity of the lead variant, scFv1, was tested with SPR and compared to that of ranibizumab and brolucizumab (Fig. 6F). It was shown that specificity of our scFv1 is similar to those of ranibizumab and brolucizumab, not binding to PLGF, VEGF-B, C or D. Moreover, scFv1 also recognizes VEGF110 and VEGF121 isoforms. Interestingly, none of them have any affinity for rabbit VEGFA.
Lead variant potently inhibits HUVEC angiogenesis. A-D) Representative images of HUVECs after treatments. (A) HUVECs do not form tubular network in absence of VEGFA, (B) 1 nM VEGFA induces formation of a HUVEC tubular network. Addition of 2 nM (C) ranibizumab or (D) scFv1 inhibits tubular network formation. (E) Plot of angiogenesis parameters. scFv1 inhibits tubular network formation more efficiently than ranibizumab. Total length, total branching length, total segment length and total branch length were calculated by 2-way ANOVA followed by Dunett’s multiple comparison test, error bars represent SD. ns: non-significant, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001. (F) Bar graph showing binding affinities of ranibizumab, brolucizumab and scFv1 to human VEGFs, VEGF isoforms and VEGFA orthologs in model organisms.
Discussion and conclusion
Anti-VEGF therapeutics revolutionized treatment of neovascular retinal diseases including the wet form of AMD. Here, we report development of three novel anti-VEGF scFvs based on the variable sequence of ranibizumab. To date, brolucizumab remains to be the only scFv developed against VEGF for use in treatment of AMD11. scFvs are amenable to less costly microbial production due to small size and no need for post-translation modifications27. Shake flask productions by P. pastoris system showed promising results which can be further improved with large-scale fermentation systems for transferring to commercial use. Both ranibizumab and brolucizumab are produced in E. coli, however, P. pastoris is a better alternative for antibody fragment production because of its more stable, higher expression patterns and secretion into the supernatant28,29.
The small molecular weight of scFvs allows for excellent retinal penetration and less systemic absorption30. Smaller antibody fragments can be cleared quickly from the bloodstream while full-length bevacizumab demonstrates greater systemic exposure resulting in a marked reduction in plasma-free VEGF31. Also, higher effective dose can be delivered leading to a possible increase in injection interval11. The effective dose of brolucizumab delivered is about 22 times more than ranibizumab10. All these studies show that small anti-VEGF antibody fragments would replace the larger traditional biotherapeutics due to their advanced properties in intravitreal therapy.
Since ranibizumab has been very successful in the clinic32, the novel antibody formats were developed by engineering the variable chain sequence of ranibizumab. Three lead variants (scFv1/2/3) were chosen among more than 30 engineered variants based on production yields, solubility, and thermal stability. The mutation sites of the three lead variants reside in ranibizumab’s VL-CL interface which is exposed in our scFv design23. The scFv format of the ranibizumab developed here (scFvWT) displayed poorer activity than the reference ranibizumab, lead mutated variants proved to perform similar or better when compared to ranibizumab in various assays. This shows that protein engineering efforts should focus more on VL-CL interface when designing scFvs from Fab or IgG formats. Successful miniaturization of an effective antibody can lead to superior bioactivity, even when compared to the starting template.
One of the widely applied tests for anti-VEGF activity is the inhibition of VEGF induced proliferation of HUVECs33. Our results showed that scFv2 and scFv3 perform very similar to ranibizumab, while scFv1 outperforms all (Fig. 2A). Next, we utilized VEGF bioassay, a very sensitive VEGFR2 activity assay to compare all variants and 3 reference molecules (bevacizumab, ranibizumab and brolucizumab)24. This assay showed that scFv1 and scFv2 together with brolucizumab have the lowest IC50 values (0.10–0.12 µg/mL) and performed the best, which was seconded by scFv3 and ranibizumab with 0.24 and 0.27 µg/mL IC50 values (Fig. 2B). Interestingly, bevacizumab and scFvWT have 0.62 µg/mL and 2.10 µg/mL IC50. This indicates that improved activity is not solely dependent on the antibody format (scFv versus full-length IgG), but the antibody engineering is of essential importance.
For testing the in vivo efficacy of the scFv1 and scFv2 lead molecules, two different tests were conducted with the embryonic zebrafish model. The subintestinal vein (SIV) formation is induced by VEGFA and inhibition of SIV formation has been shown with small molecule inhibitors of VEGFR, anti-VEGF antibody bevacizumab34,35,36. The SIV inhibition assay showed that scFv1 and scFv2 can both inhibit the endogenous VEGF expressed in the zebrafish embryo, much better than the reference molecule ranibizumab when applied at equimolar amounts (appr. 2.6 nmol per embryo) (Fig. 3). To our knowledge this is the first study where efficacy of ranibizumab is tested in the zebrafish SIV inhibition model. At tested concentration, which is at the effective concentration range reported in Zhang et al. (2018), ranibizumab did not inhibit SIV angiogenesis, while smaller scFv antibody fragments effectively inhibited SIV formation36. It is possible that ranibizumab could inhibit SIV at higher concentrations, but this was not tested in the current study. The morphological defects or death observed in scFv2 injected embryos indicated a possible toxicity of this variant, however a direct translation from fish embryonic toxicity to clinical toxicity is not possible since angiogenesis during early development is vitally important, and the lethality may be a result of high inhibitory activity. A second assay was performed to model the retinal vessel leakage observed in wet AMD patients. This was achieved by previously reported hypoxia induced with CoCl2 treatment of developing zebrafish embryo, which caused a very high molecular weight dextran to leak out of the vessels25. This assay showed that average leak was lower when either scFv1 or scFv2 were applied, however only scFv1 had a statistical significance (Fig. 5). It was previously reported that bevacizumab and ranibizumab suppresses hypoxia-induced vessel branching in the zebrafish eye, however in our hands ranibizumab did not suppress the retinal leak. Further comparison of different anti-VEGF antibodies in the zebrafish retinal leakage assay is needed to define bioactivities of various antibody formats further. However, the anti VEGF scFv1 fragment reported here suppressed retinal leakage. When all in vitro and in vivo results were evaluated, scFv1 was selected as the lead molecule for further use. Finally, the angiogenesis inhibition test showed that scFv1 is more potent than ranibizumab in inhibiting the organization of HUVECs into tubular networks (Fig. 6). Here, a comprehensive approach combining in vitro assays and in vivo zebrafish models was used to analyze the bioactivity of the novel anti-VEGF scFvs. Our candidate of scFv1 was confidently selected as the lead molecule which will be studied further for anti-VEGF therapy development.
Data availability
Raw data of all experiments are available from the corresponding author upon reasonable request.
References
-
Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676 (2003).
-
Campbell, M. & Doyle, S. L. Current perspectives on established and novel therapies for pathological neovascularization in retinal disease. Biochem. Pharmacol. 164, 321–325 (2019).
-
Fleckenstein, M. et al. Age-related macular degeneration. Nat. Reviews Disease Primers. 7, 31 (2021).
-
Perez-Gutierrez, L. & Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell. Biol. 24, 816–834 (2023).
-
Ambati, J. & Fowler, B. J. Mechanisms of age-related macular degeneration. Neuron 75, 26–39 (2012).
-
Ferrara, N. VEGF and intraocular neovascularization: from discovery to therapy. Transl Vis. Sci. Technol. 5, 10 (2016).
-
Chen, Y. et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured fab in complex with antigen. J. Mol. Biol. 293, 865–881 (1999).
-
Ferrara, N. & Adamis, A. P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 15, 385–403 (2016).
-
Heier, J. S. et al. View & Groups, V. S. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration, Ophthalmology. 119, 2537-48. (2012).
-
Holz, F. G. et al. Single-Chain antibody fragment VEGF inhibitor RTH258 for neovascular Age-Related macular degeneration: A randomized controlled study. Ophthalmology 123, 1080–1089 (2016).
-
Nguyen, Q. D. et al. Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular Age-Related macular degeneration. Ophthalmology 127, 963–976 (2020).
-
Bodaghi, B. et al. Detection and management of intraocular inflammation after Brolucizumab treatment for neovascular Age-Related macular degeneration. Ophthalmol. Retina. 7, 879–891 (2023).
-
Brown, D. M. et al. MERLIN: Two-Year Results of Brolucizumab in Participants with Neovascular Age-Related Macular Degeneration and Persistent Retinal Fluid, Ophthalmology. (2024).
-
Busch, M. et al. Anti-drug antibodies to Brolucizumab and Ranibizumab in serum and vitreous of patients with ocular disease. Acta Ophthalmol. 100, 903–910 (2022).
-
Arslan, M. et al. Effect of non-repetitive linker on in vitro and in vivo properties of an anti-VEGF ScFv. Sci. Rep. 12, 5449 (2022).
-
Baylet, A. et al. Transcutaneous penetration of a single-chain variable fragment (scFv) compared to a full-size antibody: potential tool for atopic dermatitis (AD) treatment. Allergy Asthma Clin. Immunol. 17, 73 (2021).
-
Knox, C. et al. DrugBank 6.0: the drugbank knowledgebase for 2024. Nucleic Acids Res. 52, D1265–D1275 (2024).
-
Kalyoncu, S. et al. Process development for an effective COVID-19 vaccine candidate harboring Recombinant SARS-CoV-2 delta plus receptor binding domain produced by Pichia pastoris. Sci. Rep. 13, 5224 (2023).
-
Abad, S. et al. Real-time PCR-based determination of gene copy numbers in Pichia pastoris. Biotechnol. J. 5, 413–420 (2010).
-
Sinha, J. et al. Cell bank characterization and fermentation optimization for production of Recombinant heavy chain C-terminal fragment of botulinum neurotoxin serotype E (rBoNTE(H(c)): antigen E) by Pichia pastoris. J. Biotechnol. 127, 462–474 (2007).
-
Lawson, N. D. & Weinstein, B. M. In vivo imaging of embryonic vascular development using Transgenic zebrafish. Dev. Biol. 248, 307–318 (2002).
-
Carpentier, G. et al. Angiogenesis analyzer for ImageJ – A comparative morphometric analysis of endothelial tube formation assay and fibrin bead assay. Sci. Rep. 10, 11568 (2020).
-
Koenig, P. et al. Mutational landscape of antibody variable domains reveals a switch modulating the interdomain conformational dynamics and antigen binding. Proc. Natl. Acad. Sci. U S A. 114, E486–E495 (2017).
-
Wang, L. et al. Development of a robust reporter-based assay for the bioactivity determination of anti-VEGF therapeutic antibodies. J. Pharm. Biomed. Anal. 125, 212–218 (2016).
-
Wu, Y. C. et al. Hypoxia-induced retinal neovascularization in zebrafish embryos: a potential model of retinopathy of prematurity. PLoS One. 10, e0126750 (2015).
-
Liu, L. et al. A novel engineered VEGF blocker with an excellent Pharmacokinetic profile and robust anti-tumor activity. BMC Cancer. 15, 170 (2015).
-
Marty, C., Scheidegger, P., Ballmer-Hofer, K., Klemenz, R. & Schwendener, R. A. Production of functionalized single-chain Fv antibody fragments binding to the ED-B domain of the B-isoform of fibronectin in Pichia pastoris. Protein Expr Purif. 21, 156–164 (2001).
-
Spadiut, O., Capone, S., Krainer, F., Glieder, A. & Herwig, C. Microbials for the production of monoclonal antibodies and antibody fragments. Trends Biotechnol. 32, 54–60 (2014).
-
Krishna, S., Jung, S. T. & Lee, E. Y. Escherichia coli and Pichia pastoris: microbial cell-factory platform for -full-length IgG production. Crit. Rev. Biotechnol. 45, 191–213 (2025).
-
Uludag, G. et al. Efficacy and safety of intravitreal anti-VEGF therapy in diabetic retinopathy: what we have learned and what should we learn further? Expert Opin. Biol. Ther. 22, 1275–1291 (2022).
-
Avery, R. L. et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or Aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 98, 1636–1641 (2014).
-
Holz, F. G. et al. Safety of Ranibizumab in routine clinical practice: 1-year retrospective pooled analysis of four European neovascular AMD registries within the LUMINOUS programme. Br. J. Ophthalmol. 97, 1161–1167 (2013).
-
Carneiro, A. et al. Comparative effects of bevacizumab, Ranibizumab and Pegaptanib at intravitreal dose range on endothelial cells. Exp. Eye Res. 88, 522–527 (2009).
-
Serbedzija, G. N. & E., F. & C.E., W Zebraash angiogenesis: A new model for drug screening. Angiogenesis 3, 353–359 (1999).
-
Ma, C. et al. A systematic comparison of anti-angiogenesis efficacy and cardiotoxicity of receptor tyrosine kinase inhibitors in zebrafish model. Toxicol. Appl. Pharmacol. 450, 116162 (2022).
-
Zhang, J., Gao, B., Zhang, W., Qian, Z. & Xiang, Y. Monitoring antiangiogenesis of bevacizumab in zebrafish. Drug Des. Devel Ther. 12, 2423–2430 (2018).
Acknowledgements
Authors thank Izmir Biomedicine and Genome Center for providing the infrastructure and personel support required for this project. We thank IBG Optic Imaging Core Facility and Zebrafish Facility staff for providing technical support. Authors thank Merve Basol for her support in statistical analysis. GC-A: Wrote the main manuscript text, designed study, revised all figures, OE: conducted cell culture experiments and prepared figures, CO: Cloned and produced antibodies, EO:conducted zebrafish experiments, prepared figures, OM: purified antibodies, prepared figures, SG: cloned and produced antibodies, MA and MEA: Conducted SPR analysis, MI: Designed study, SK: Designed Study, acquired funding, revised manuscript.
Funding
This study was funded by VSY-Biotechnology to IBG center under Grant no IBG002. Ceren Ozer and Seyda Gullu were funded by IBG002. Ebru Onal was funded by TUBITAK119Z161 and IBG002. Ozlem Erez was funded by IBG002 and European Union RareBoost Project #952346. Merve Arslan was funded by TUBITAK119Z16, TUBITAK-BIDEB 2211A PhD Achievement Grant and Council of Higher Education (YÖK) 100/2000 Fellowship Program.
Ethics declarations
Patent disclosure
The three scFv molecules reported here were filed for patenting under PCT/TR2022/050990.
Ethics Disclosure
Zebrafish experiments were conducted in embryos or larvae prior to independent feeding stage. According to EU Directive 2010/63, this stage is exempt from ethical regulations. All animal experimentation was conducted in accordance with EU Directive 2010/63 and national regulations. Animal experimental methods are reported in accordance with ARRIVE guidelines.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cakan-Akdogan, G., Erez, O., Ozer, C. et al. Novel anti-VEGF scFv antibodies with superior in vitro and in vivo activities. Sci Rep 15, 28009 (2025). https://doi.org/10.1038/s41598-025-11406-y
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-025-11406-y