Chief Technical Officer
Adcendo
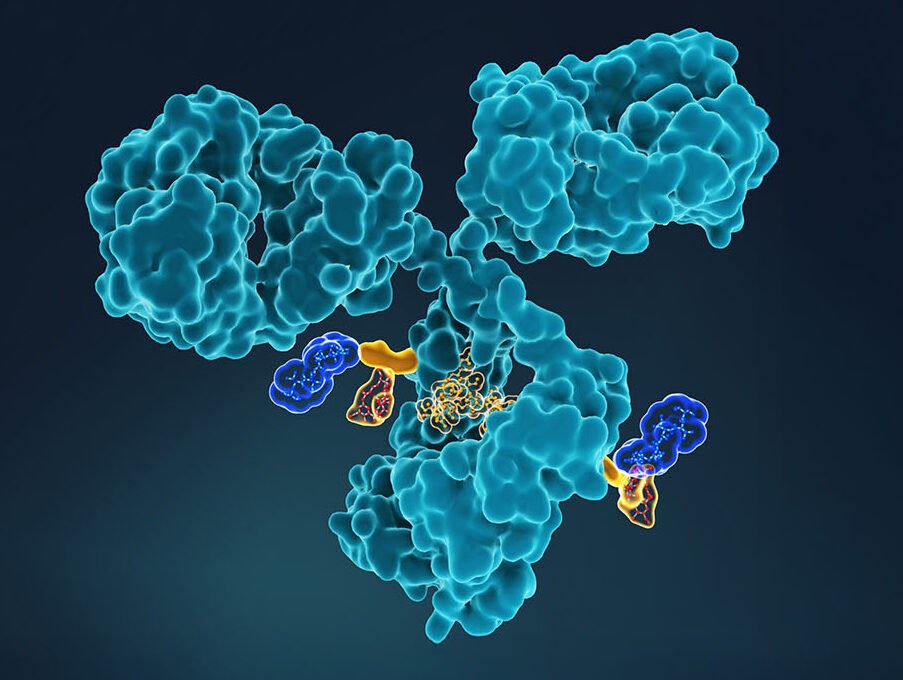
Antibody-drug conjugates (ADCs) link cancer drug payloads, such as tubulin inhibitors and DNA damaging agents, to monoclonal antibodies that selectively target tumor cells. Pernille Hemmingsen, PhD, CTO of Adcendo, says the overarching ambition of ADC technology is to improve upon the untargeted, unprecise, and highly toxic effects of chemotherapy, which doesn’t distinguish between healthy and diseased tissues.
“We are witnessing a paradigm shift for cancer treatment, where ADCs are replacing chemotherapy as new standard of care in many hard-to-treat solid tumor indications. Being part of that important improvement for cancer patients is what drives me and the whole team,” Hemmingsen told GEN.
A Copenhagen-based clinical stage ADC company, Adcendo was founded in 2017. The company’s lead program targets Tissue Factor (TF), an ADC target that is highly overexpressed in multiple epithelial cancers. A Phase I study of ADCE-T02, a topoisomerase-1 inhibitor-based ADC targeting TF, is enrolling in the US and Australia. The company’s second clinical program targets uPARAP, an endocytic receptor that is highly overexpressed in mesenchymal cancers. A Phase I study of ADCE-D01, the company’s uPARAP ADC candidate, is enrolling patients in the US and Europe.
As of March 2024, 13 ADCs have received Food and Drug Administration (FDA) approval, with more than 100 potential ADC drugs at different stages of clinical trials (see Table 1). This ADC momentum has its roots in advances in biological technologies, including effective antibody/payload pairings.

One well-known example from the FDA-approved list is the blockbuster Enhertu, an ADC-based therapeutic developed by AstraZeneca and Daiichi Sankyo. Enhertu chemically links trastuzumab, a monoclonal antibody that targets HER2 (a protein that plays a key role in normal cell growth and repair and is upregulated in many cancers, particularly breast cancer), with the topoisomerase I inhibitor payload, deruxtecan.

The ADC formulation expands trastuzumab’s therapeutic effect to HER2-low breast cancer by eliciting a bystander effect, where the deruxtecan warhead diffuses into tumor antigen-negative cells to allow Enhertu to be a suitable therapy for cancers with heterogenous HER2 expression. The effect also enables the killing of non-cancer tumor elements, such as stromal or vascular cells, further increasing the tumor-killing potential.
Enhertu first received FDA approval in December 2019 for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer. It achieved blockbuster status in 2022, with global sales exceeding $1.6 billion. In April 2024, the drug was approved in the U.S. as the first tumor-agnostic HER2-directed therapy for previously treated patients with metastatic HER2-positive solid tumors.
Complex links
While many companies pursue ADC candidates based on the context of the clinical indication, Switzerland-based Araris Biotech has focused on developing its own ADC-payload linker technology. While standard ADCs carry one payload on each of the two heavy chains of the antibody, Araris’s novel linker, known as AraLinQ, introduces branches to allow the addition of multiple payloads in a one-step conjugation process.
Araris was founded in 2019 and was acquired by Taiho Pharmaceuticals for $1.14 billion this past March. The biotech also announced a handful of partnerships earlier this year, including a collaboration with Roche’s Chugai Pharmaceuticals that is potentially worth over $780 million.

Chief Technical Officer
Araris
According to Isabella Attinger-Toller, PhD, CTO of Araris, ADCs that only carry a single payload class can face challenges in drug resistance and tumor heterogeneity. For example, single cells within a tumor can vary by cell type and sensitivity to the drug. On the molecular level, differences in cell receptor density can impact ADC targeting efficacy.
“Combining modes of action is an important approach that weakens tumor cells from two different sites,” she told GEN. “Our platform’s versatility allows for the combination of at least a dozen payload classes to enable rapid optimization for various cancer types.”
Notably, AraLinQ takes advantage of off-the-shelf antibodies that do not require additional engineering that may introduce stability or developability challenges. However, ADC manufacturing remains a complex task for the field as a whole.
CDMOs and AI increasingly engaged

Senior Director, Lonza
According to Iwan Bertholjotti, senior director of commercial development and marketing strategy bioconjugates at Lonza, 78% of manufacturing services are outsourced to experienced CDMOs that have established expertise in process development and evaluation of quality attributes.
“Supply chains are complicated. Successful CDMOs bring it all under one roof. Integrated solutions is a big topic where you can support both early-stage biotechs and big pharma companies to overcome supply chain challenges,” Bertholjotti told GEN.
Lonza has taken to artificial intelligence (AI) to augment its ADC manufacturing capabilities. In a collaboration announced in November 2024, Synaffix, an ADC company acquired by Lonza in 2023, licensed its technology to BigHat Biosciences, a Bay Area-based biotech company leveraging AI to design antibody therapeutics.

Under the terms of the agreement, BigHat receives target-specific access to Synaffix’s late-clinical stage ADC technology platform. Lonza will also provide access to its DNA-to-IND offering, which allows drug developers to receive the necessary cGMP/non-cGMP ADC batches leading up to the first clinical trial. The full stack encompasses the production of the antibody, bioconjugation, and drug product filling.
Targeted therapy in combo
ADCs are part of a growing toolbox of targeted cancer therapies, which includes immune checkpoint inhibitors and CAR T-cell therapies, among others. Many companies are taking a combination approach to improve patient outcomes.
Agenus is a clinical-stage immunotherapy company whose lead immuno-oncology combination, botensilimab (BOT) and balstilimab (BAL), has shown clinical responses across nine metastatic, late-line cancers after evaluation in more than 1,200 patients across Phase I and Phase II clinical trials. BOT is a human Fc-enhanced CTLA-4 blocking antibody designed to boost both innate and adaptive anti-tumor immune responses, while BAL is an investigational PD-1 antibody.
Zack Armen, head of investor relations, corporate development at Agenus, shared single-patient examples that demonstrated complete solid tumor eradication after a few weeks of BOT/BAL treatment. The results, while promising, were only seen in a subset of patients.
In June, Agenus announced a research collaboration with Noetik, an AI-focused multimodal biology company, to identify actionable biomarkers that can predict which patients are most likely to benefit from BOT/BAL treatment using Noetik’s virtual cell model, OCTO. Insights from Noetik’s AI models aim to inform the design of BOT/BAL’s Phase III clinical trial.
“What we hope to see in our work with Noetik is raising that complete tumor eradication rate from 30–35% to, eventually, 60%,” said Armen. “If we add in another therapy and Noetik is able to build another model using that triplet combination, maybe we can break into 70–80%.”
OCTO is composed of 1.5 billion parameters and is trained on Noetik’s proprietary multimodal datasets, which span spatial proteomics, spatial transcriptomics, H&E (hematoxylin and eosin) pathology, DNA genotyping, and clinical metadata from nearly 200 million tumor and immune cells collected from thousands of patients with cancers such as colorectal cancer, non-small cell lung cancer, ovarian cancers, and sarcomas.
“We tend to spend a lot of time asking, ‘How do I find a better drug for these targets? How do I tackle undruggable targets and develop better molecules?’” Ron Alfa, MD, PhD, CEO of Noetik, told GEN. “The vision behind Noetik is developing models that understand patient biology and predict which drugs are going to work in which patients.”
Taken together, the promise of more efficacious targeted therapies for cancer will be realized from the interplay between rising biological, chemical, and computational technologies. A paradigm shift in cancer treatment continues to unfold as the new wave of ADC candidates march toward clinical approval.
The post Making New Connections: Antibody Drug Conjugates Target Cancer appeared first on GEN – Genetic Engineering and Biotechnology News.