FDA-approved Alzheimer’s immunotherapies have shown some benefit, but serious side effects and poor brain delivery continue to pose challenges. The newly designed antibody transport vesicle produced by Michelle Pizzo, PhD, from Denali Therapeutics, and colleagues at Denali and Genentech, holds promise as a potential solution to these challenges.
The team detailed their work in a paper published in Science titled, “Transferrin receptor-targeted anti-amyloid antibody enhances brain delivery and mitigates ARIA.”
A functional hallmark of Alzheimer’s disease is the buildup of amyloid-β (Aβ) protein plaques in the brain. Current anti-amyloid drugs like aducanumab, lecanemab, and donanemab are approved to treat Alzheimer’s disease by targeting Aβ plaques in the brain. While these treatments can slow cognitive decline and reduce Aβ plaque buildup, the drugs are not a cure, and they have limited ability to halt or reverse the disease symptoms. Further, the side effects of this treatment are potentially serious, including brain swelling and microbleeds in the brain, which are collectively referred to as amyloid-related imaging abnormalities (ARIA).
To address both efficacy and safety limitations, the researchers explored receptor-mediated transport across the blood-brain barrier (BBB). This strategy utilizes native transport proteins to convey the therapeutics between the vascular system and the brain. They targeted the transferrin receptor (TfR), a protein well known for its functional ability to transport some molecules across the BBB.
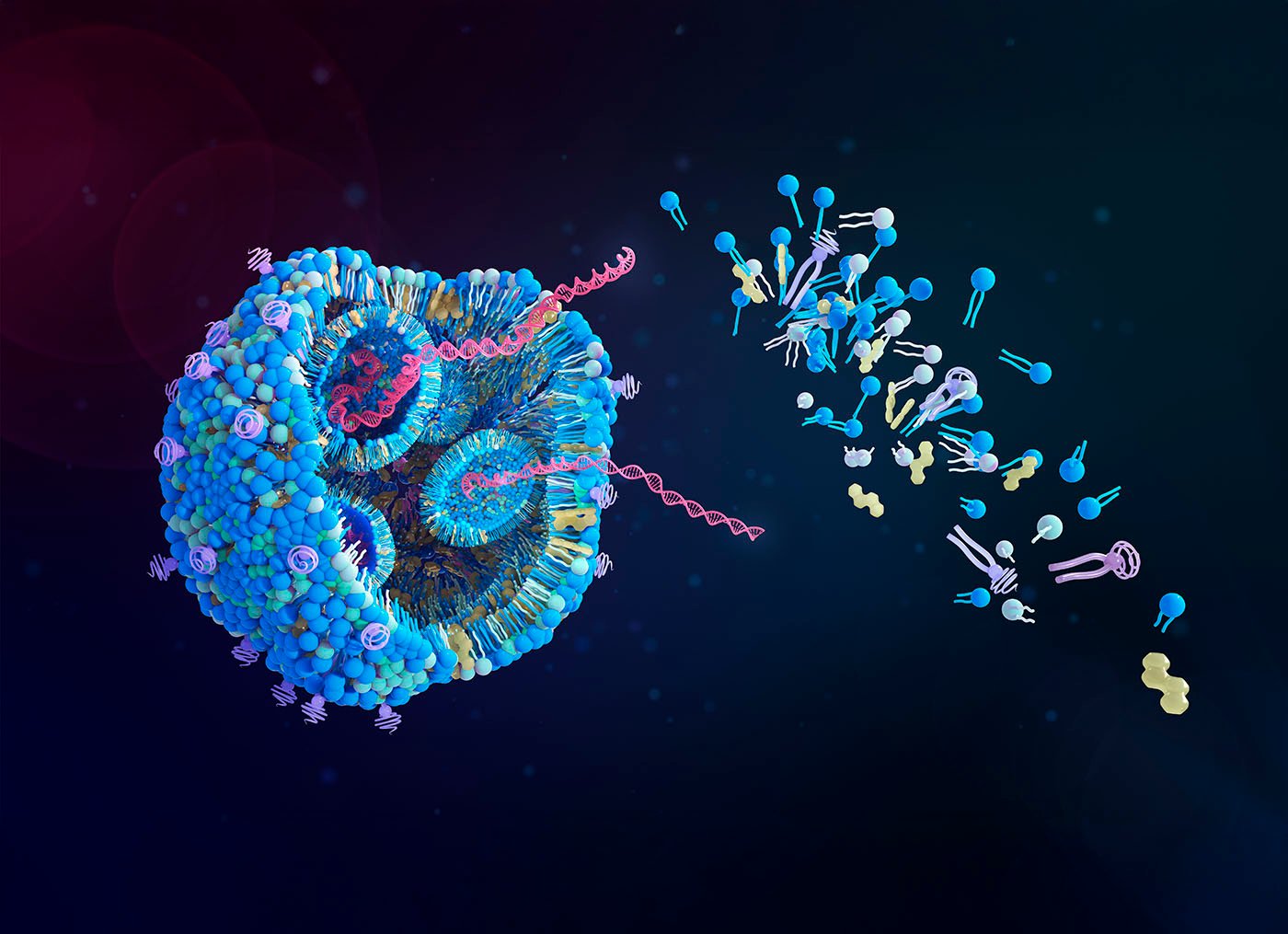
“We engineered an antibody transport vehicle (ATV) with asymmetrical Fc mutations (ATVcisLALA) that mitigated TfR-related hematology liabilities,” wrote the authors. They further explained that this mutated ATV maintains the function of clearing the Aβ plaques while also reducing interactions with immune cells and immature red blood cells.
The researchers then fused the transporter to an anti-Aβ antibody to create ATVcisLALA:Aβ. In mice, the modified transporter successfully entered and penetrated brain tissue more efficiently than conventional antibodies. Compared to conventional anti-Aβ antibodies, the modified therapy achieved 5- to 8-fold higher concentrations in brain tissue, with broader distribution across the parenchyma and significantly reduced accumulation around blood vessels.
While traditional anti-Aβ antibodies tend to accumulate near arteries—triggering vascular inflammation and contributing to ARIA—the ATVcisLALA:Aβ construct primarily crossed capillaries, reducing inflammatory responses.
In fact, mice treated with the engineered therapy showed near-complete elimination of ARIA-like lesions and vascular inflammation.
“Administration of ATVcisLALA:Aβ in mice exhibited broad brain distribution and enhanced parenchymal plaque target engagement,” wrote the authors. “This biodistribution reduced ARIA-like lesions and vascular inflammation.”
The work demonstrates that it may be possible to preserve both brain penetration and immune effector function in a single therapeutic design. That combination could be critical for next-generation anti-Aβ therapies, balancing efficacy and safety.
“ATVcisLALA has the potential to improve the next generation of Aβ immunotherapy, through enhanced biodistribution mediated by transport across the BBB,” concluded the authors.
In a related Perspective, Mengen Xing, PhD, and Weihong Song, PhD, both of Wenzhou Medical University, Wenzhou, Zhejiang, China, concurred: “Taken together, the findings of Pizzo et al. provide more than preclinical proof of concept; they also establish a framework for therapeutic design.”
The post Alzheimer’s Therapy Delivery Boosted with Engineered Antibody appeared first on GEN – Genetic Engineering and Biotechnology News.