Researchers at the Laboratory of Psychiatry and Experimental Alzheimer’s Research, Department of Psychiatry and Psychotherapy, Medical University of Innsbruck, have developed a low-cost and easy-to-use model for studying living brain cells in Alzheimer’s disease and Parkinson’s disease. The new approach uses novel “ring-inserts” that allow the long-term culture and imaging of nerve cells directly in brain tissue, offering a new platform for drug testing, live-cell microscopy, and disease research.
The team, headed by Christian Humpel, PhD, reported on a study in which they provided evidence that cholinergic and dopaminergic neurons survived on the “ring-inserts” for at least two weeks. They further microcontact printed onto the “ring-inserts” nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF), and visualized the growth of cholinergic and dopaminergic neurons along the tracks.
The researchers suggest that the “ring-insert” system provides a cost-effective, scalable, and ethically responsible platform for studying the living brain. By combining realistic tissue models with live cell imaging capabilities, the technology opens new doors in neuroscience, disease modeling, and drug development, bringing researchers closer to understanding and treating the brain’s most complex disorders.
In their paper published in Biofunctional Materials, titled “Organotypic mouse brain slices: low-cost “ring-inserts” to study cholinergic and dopaminergic neurons with live cell imaging with an emphasis on calcium imaging,” senior author Humpel and colleagues, stated, “The ‘ring-insert’ represents a low-cost and easy model for culturing organotypic brain slices and allows for subsequent live-cell imaging.”
Alzheimer’s and Parkinson’s disease are among the most devastating neurodegenerative disorders, affecting millions of people worldwide. They progressively destroy specific types of brain cells, leading to memory loss, movement difficulties and severe cognitive decline. To understand how and why neurons die in Alzheimer’s and Parkinson’s diseases, and to help develop substances that can protect or regenerate them, reliable experimental models are essential.
One promising approach is the use of organotypic brain slice cultures. These are 150 µm-thick sections of brain tissue, about the width of a human hair, taken from young mice.
These slices preserve the brain’s natural structure and organization, allowing scientists to study living nerve cells in a realistic environment outside the body. Such organotypic brain slices “… bridge the gap between in vitro cell cultures and in vivo animal experiments,” the investigators wrote. “In contrast to homogeneous single-cell cultures, the complex 3D architecture of the brain is preserved in organotypic brain slice cultures, thus allowing for the simulation of more in vivo-like situations.” Given that the main architecture of the cells is preserved, it allows for the in vitro investigation of cellular and molecular processes in the brain areas of interest.”
Such models are not only important for neuroscience research but also contribute to the so-called 3R principle in animal research, which is to reduce the number of animals used, refine methods to minimize harm, and replace animal experiments wherever possible. From a single mouse brain, around 50-100 slices can be obtained, significantly reducing the number of animals required.
However, culturing brain slices remains technically demanding—“this technique is highly complex and requires months of experience to prepare excellent slices”—the authors continued, and the slices cannot be used for live-cell imaging. “… the visualization of brain cells in multilayer organotypic slices using live-cell imaging for extended periods is difficult,” they noted. The technology is also expensive, especially when using commercial membrane inserts, which can cost up to 20 euros a piece and often limit the ability to observe living cells under the microscope. For example, a typical experiment using 200 brain slices would cost between €3,000 and €4,000 with commercial inserts.
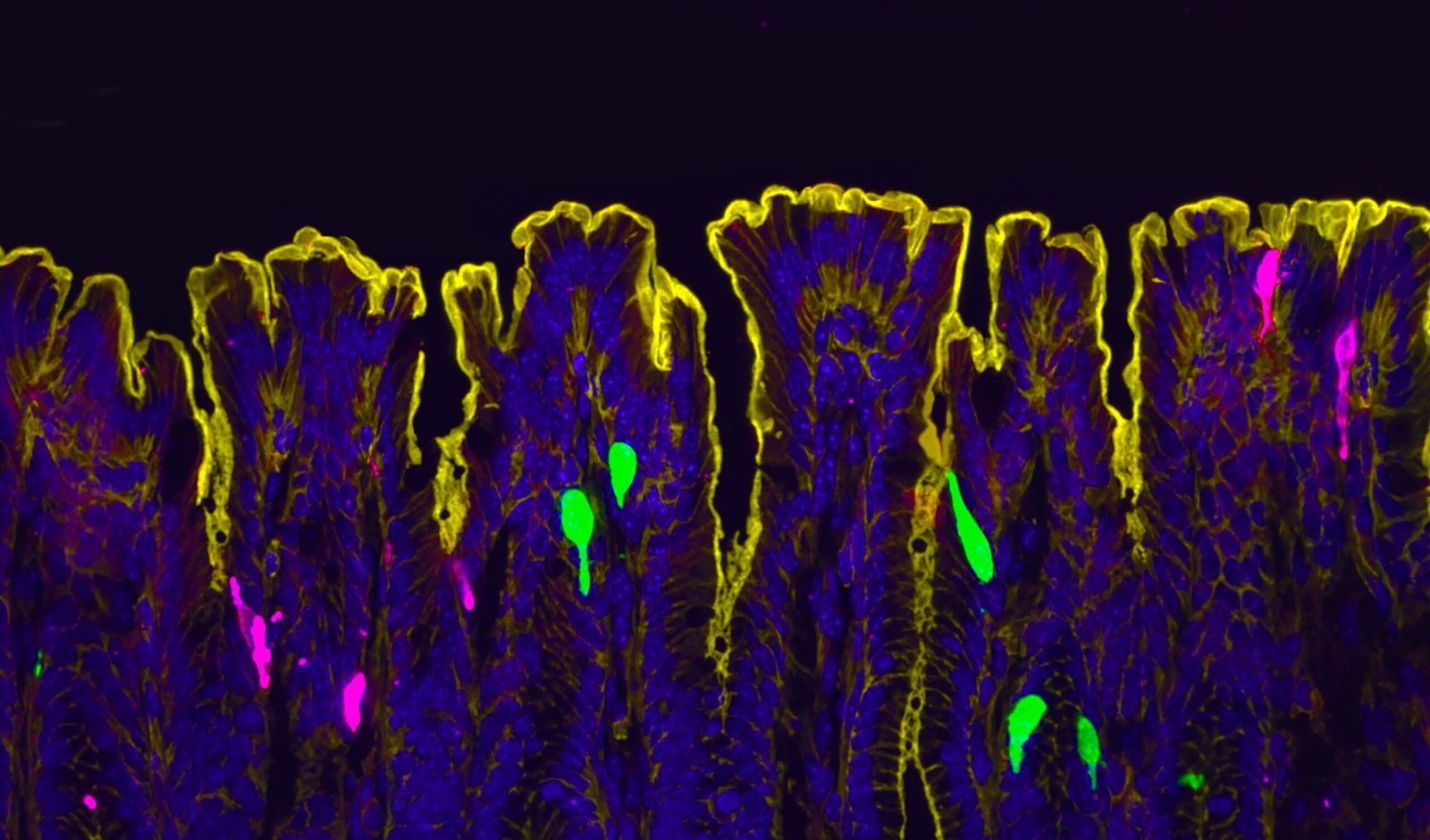
To address these drawbacks, Humpel and team developed a simple and innovative solution in the form of self-made “ring-inserts.” Each insert consists of a small silicone ring with a thin, permeable membrane attached to its base. “… we created a ‘ring-insert’ by gluing a low-cost (roll) membrane on plastic rings,” the investigators explained. Thin brain slices are placed directly onto this membrane, which sits just above a reservoir of nutrient-rich culture medium. The membrane allows nutrients to diffuse up into the tissue, keeping the slices alive for extended periods. Because the tissue lies flat and remains stable during cultivation, the inserts can be used directly for microscopic imaging of the living brain cells. “These ‘ring-inserts’ can be cultured with organotypic coronal mouse brain slices (150 μm) on top with the well-established interface method,” the investigators added. “In addition, these ‘ring-inserts’ can be easily inverted and visualized under an inverse fluorescence microscope for live-cell imaging.”
Humpel added, “Our ring-inserts cost only about two to three euros each, making them up to ten times more affordable than standard commercial inserts. Despite their simplicity, they support high-quality brain tissue cultures and allow us to observe living neurons in real time, including how they grow, form connections and respond to stimulation.”
The same setup would cost only around €500 using the self-developed “ring-inserts.” This represents a potential cost reduction of up to €3,500 without compromising experimental quality.

In their reported study the researchers focused on two key types of nerve cells involved in neurodegenerative diseases. These are cholinergic neurons—which help regulate memory and attention and are severely affected in Alzheimer’s disease—and dopaminergic neurons, which play a central role in movement and are lost in Parkinson’s disease.
To guide the growth of new nerve fibers, the team used a technique called microcontact printing (µCP), which involves stamping tiny lines of growth factors onto the membrane surface of the “ring-inserts.” These lines act like tracks, or paths, that direct the growth of nerve fibers.
Growth factors are naturally occurring proteins that support the survival, growth, and differentiation of neurons. Specifically, the researchers printed NGF, which promotes the development of cholinergic neurons, and GDNF, which supports dopaminergic neurons. After printing, the organotypic brain slices were cultured directly on these patterned “ring-inserts,” allowing the neurons to grow along the predefined lines. Both cell types were successfully cultured on the “ring-inserts” and survived for two weeks.
Using these “ring-inserts,” the researchers were not only able to keep cholinergic and dopaminergic neurons alive in culture but also to study their structure and activity in detail. The inserts proved ideal for live-cell imaging, making it possible to observe individual neurons and their growing nerve fibers over time. To test whether the cells were still functionally active, the team used a method called calcium imaging. They added fluorescent dyes, such as Rhod-4 and Fluo-4, that light up when calcium enters the cell, a signal that the neuron is responding to a stimulus. After applying a chemical trigger (potassium chloride), the researchers observed clear flashes of fluorescence, showing that the neurons were not only intact but also capable of functional activity and communication.
“We can monitor live neuronal activity in real time,” said first author Alessa Gern, PhD. “It gives us direct insight into how these cells function, respond to stimulation and potentially degenerate. This was not possible before with such cost-effective and simple tools like the ‘ring-inserts’.”
The researchers also see future applications beyond basic neuroscience. Beyond cost and ethical advantages, the “ring-insert” model is highly versatile. It can be combined with drug testing, genetic engineering, viral delivery, or high-resolution imaging techniques. The inserts can also be modified to support more complex co-culture systems, such as combining brain slices with blood vessel cells to simulate the blood–brain barrier, a key structure for studying how substances enter or are blocked from the brain.
Looking ahead, the team mentions that the method could also be adapted for use with adult brain tissue or even with human samples obtained from surgical procedures. This would bring laboratory models even closer to real disease conditions and could help accelerate the discovery of new therapies for Alzheimer’s, Parkinson’s, and related disorders.
“Our vision is to enable long-term, real-time observation of living brain cells in a flexible and affordable setup,” suggested Humpel. “This could help transform how we study the brain and how we develop treatments, while also supporting the move away from animal experiments.”
The post Alzheimer’s and Parkinson’s Research Advanced with Low-Cost “Ring-Inserts” appeared first on GEN – Genetic Engineering and Biotechnology News.