- Review
- Open access
- Published:
- Yucong Geng1,
- Alishba Shaukat2,
- Wania Azhar2,
- Qurat-Ul-Ain Raza3,
- Ayesha Tahir2,
- Muhammad Zain ul Abideen4,
- Muhammad Abu Bakar Zia5,
- Muhammad Amjad Bashir1 &
- …
- Abdur Rehim6
Biotechnology for Biofuels and Bioproducts volume 18, Article number: 93 (2025) Cite this article
Abstract
This review critically examines the entire value chain of microalgal biorefineries, with the central aim of elucidating the key technological, economic, and environmental enablers and barriers that govern their transition from pilot-scale demonstrations to commercially viable, circular-economy applications. A systematic literature search was conducted across five major scientific databases using predefined Boolean strings: “algal biorefineries,” “microalgae biofuel,” “techno-economic analysis,” “life-cycle assessment,” and “bioproduct recovery.” Inclusion criteria encompassed peer-reviewed studies and authoritative policy documents published between January 2007 and March 2025 that provided empirical data on upstream cultivation, midstream processing, and downstream conversion, as well as techno-economic assessments (TEA) and life-cycle analyses (LCA). Exclusion criteria included non-English commentaries, purely theoretical models without experimental validation, and studies that focused exclusively on single-product streams. Unlike previous reviews that address isolated segments of the algal biorefinery pipeline, this work delivers a novel, integrative framework that synthesizes recent advances across cultivation modes, genetic and metabolic engineering, AI‐enabled optimization, and IoT‐driven monitoring. This review critically evaluates the trade-offs between CAPEX and OPEX, energy penalties associated with harvesting and drying, and inconsistencies in LCA to identify, where performance improvements yield the most significant economic and environmental returns. Finally, this review proposes a targeted research roadmap, spanning multivariate strain engineering, hybrid cultivation architectures, low‐energy harvesting technologies, cascade‐compatible fractionation platforms, standardized LCA/TEA protocols, and supportive policy mechanisms, that charts a clear path toward overcoming current bottlenecks. This comprehensive, evidence‐based synthesis aims to inform both academic research and industrial strategy, thereby advancing the field of sustainable algal biorefineries.
Introduction
Energy is a cornerstone of socio-economic development, whose demand has been increasing rapidly due to urbanization, economic growth, and population extension. Conversely, conventional energy sources are not viable in the long run from either an economic or an ecological point of view [83]. The energy return on investment for oil and gas is experiencing a decline. At the same time, fossil fuels remain at the top in contributing to greenhouse gas emissions (GHG), which account for 87% of all CO2 and GHG emissions across the globe [40, 60]. GHG-induced climate change has caused irreversible damage, such as biodiversity loss, rising sea levels, and oceanic acidification [120]. Because CO2 is long-lived in the atmosphere, a drastic reduction in emission rates is urgently required to stabilize its concentration below 550 parts per million, including the cessation of using fossil fuels in developed countries by 2050 [127]. The growing dependence on exhaustible resources calls for the urgent adoption of sustainable alternatives; biofuels are better placed considering their low net CO2 emission and compatibility with existing infrastructure [43].
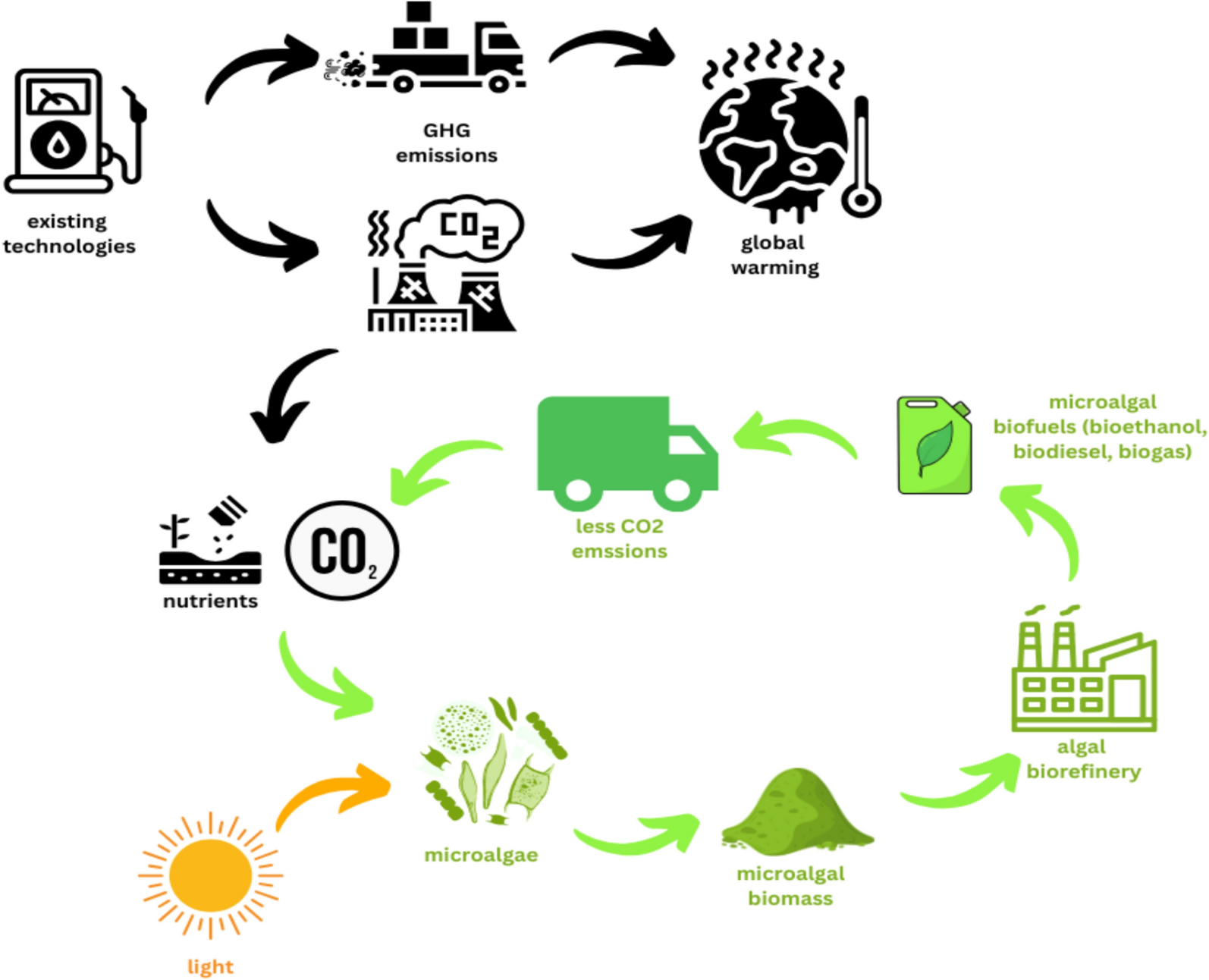
Algae have gained significant interest as a potentially viable feedstock for good biogenic fuel production and other augmented side products. Algae differ from conventional agricultural feedstocks due to attribution to their high proliferation rates, potentially high lipid composition, and aptitude for growth under various conditions on uncultivatable land and in wastewater, hence least competing with consumer food supplies [19]. Consequently, with such high photosynthetic efficiency, large amounts of CO2 could be captured, making it a potentially important tool for mitigating greenhouse gas emissions, as shown in Fig. 1 [13]. Besides, algae are composed of a variety of biomolecules for instant proteins, lipids, and carbohydrates. The products are used to produce bioenergy, pharmaceutical products, and aquaculture feed amongst others [19]. Preceding parameters make algae a kind of multi-functional feedstock manners that ensure energy safety and environmental challenges. The algal biorefinery is a designed, integrated production system of the various coveted commodities of bio-based fuels, biochemicals, and biomaterials through the feedstock of the algal biomass with focus on maximum resource efficiency [52].
Biorefinery is parallel to the concept of a petroleum refinery, but it is based on renewable biomass feedstock [35]. Because of its high productivity and diverse biochemical composition, algal biomass can be fractionated into several valuable components: lipids for biodiesel production, carbohydrates for bioethanol, and proteins for animal feed or nutraceuticals [18]. The integrated approach will surely make a given biomass fraction reach its maximum value, where the generation of waste is at a minimum. This is because residues have other uses for bioenergy uses or as applications for soil amendments [54]. For example, water and nutrients are recycled during production, which reduces the environmental impacts of that process [120]. Biorefineries of this sort will set up a sustainable route toward meeting energy and material demand by contributing to the environment’s conservation through the latest applied techniques of processing.
Biorefineries based on algae feedstock play are vital to maintaining energy security and environmental sustainability. Herein, from an energy perspective, algae will be used as feedstock in the biorefinery to develop a renewable and scalable solution for meeting the upward-surge global energy demand [17]. Value-added yields of various biofuels, viz., biodiesel, bioethanol, and biogas, extracted from this feedstock could replace the depleting fossil fuel reserve and limit reliance on exhaustible sources of energy [13]. The versatility of algal biomass allows the manufacturing of various co-products, such as bioplastics, pharmaceuticals, and animal feed while bringing greater economic viability to biofuel production [140]. In terms of the environmental impacts, algal biorefineries are said to contribute a lot toward reducing GHG emissions. Large amounts of CO2 are sequestered through growth, resulting from the high photosynthetic efficiency fact that balances derived emissions from biofuel combustion [19]. Algae show cultivability on non-farm land or wastewater and brackish water without struggling for a supply of freshwater and arable land. The enclosed system represents resource management well and aligns with the circular-economy concept. Therefore, algal biorefineries have become the transformative solution for securing energy supply and being more environmentally friendly.
Recent innovations and the surging interest in research in algal biorefineries have indicated their potential to become a cornerstone in sustainable solutions for the bioeconomy. Innovations within the area of algae cultivation, in terms of high-yielding varieties as well as the optimized design of photobioreactors, have substantially augmented the productivity of the biomass and efficiency of the applied resources [2]. Metabolic engineering has now involved enhancing the content of algae for lipids, carbohydrates, and proteins with biofuel and bioproduct applications in mind [70]. In recent times, introducing various different biorefinery processes has enabled the methodical redevelopment of algal biomass into everything ranging from biofuels to bioplastics and biofertilizers via anaerobic digestion, transesterification, and hydrothermal liquefaction. Increasing concern for climate change and depleting fossil fuels has greatly stimulated huge investments and policy assistance toward research in algal biorefineries. These ensure, through national and international initiatives, such as the U.S. Department of Energy’s Algae Program and the Horizon 2020 projects of the European Union, that combined efforts are effectively made toward upscaled commercialization of the algal biorefinery technologies [137]. Excluding that, the paradigm of the waste-free economy increased the interest in using algae for environmental remediation, including effluent handling and carbon bio-sequestration. In the future, algal biorefineries will play an even more important part in the sustainability framework regarding energy and materials, with continuous technological and economic challenges being researched.
Aims and novel contribution
This review addresses a critical gap in the literature by offering an integrated, end-to-end appraisal of microalgae biorefinery systems. Its principal objectives are to:
- 1.
Synthesize recent advances across the microalgal biorefinery value chain, including strain improvement, cultivation modalities (photobioreactors versus open ponds), and downstream conversion pathways for biodiesel, bioethanol, biogas, and hydrogen.
- 2.
Critically evaluate techno-economic and environmental performance, drawing on techno-economic analyses (TEA) and life-cycle assessments (LCA) to identify process configurations that optimize both cost-effectiveness and sustainability.
- 3.
Elucidate emerging technological enablers, such as genetic and metabolic engineering strategies, artificial intelligence-driven optimization, and Internet of Things (IoT)-based monitoring, and assess their potential to overcome current scalability and economic barriers.
- 4.
Propose an integrative roadmap for commercialization, emphasizing co-integration with wastewater treatment and the formulation of supportive policy frameworks to facilitate the transition from pilot-scale demonstrations to sustainable, circular-economy applications.
Methodology
Review design
This study employs a narrative literature review framework, structured according to the IMRaD (Introduction–Methods–Results–Discussion) format, which is well-suited for synthesizing heterogeneous evidence and drawing critical insights rather than conducting statistical synthesis [4]. Narrative reviews offer the flexibility necessary to integrate diverse technological, economic, and policy perspectives in algal biorefineries while maintaining methodological transparency.
Search strategy
A systematic and reproducible search protocol was designed in consultation with subject librarians:
-
Databases: PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar.
-
Search terms (using Boolean operators): (“algal biorefineries” OR “microalgae biofuel” OR “algal biodiesel” OR “algal bioethanol” OR “algae bioplastics”) AND (“photobioreactor” OR “techno-economic” OR “life-cycle analysis” OR “economic feasibility”).
-
Date range: January 2007 to March 2025, with emphasis on 2019–2025 to capture recent innovation.
-
Language: English.
-
Grey literature: Included key government and institutional reports (e.g., DOE, EU Horizon project documentation), ensuring broader context beyond peer-reviewed journals.
Each search strategy was fully documented, listing databases, date executed, exact search string, and number of retrieved records.
Inclusion and exclusion criteria
Articles were screened based on these explicit criteria, Table 1.
Data extraction and synthesis
Key data elements were extracted into a structured table, including:
|
General metadata |
Author, year, journal, country |
|---|---|
|
Technical parameters |
Algal species, cultivation method, reactor type, product type, and yield metrics |
|
Economic and environmental indicators |
Cost-per-unit, energy balance, life-cycle emissions, scale (lab/pilot/commercial) |
|
Analytical insights |
Identified barriers, enablers, and future research directions |
Synthesis was conducted thematically, organizing results into major domains, cultivation, conversion, co-product valorization, techno-economic aspects, and sustainability. Within each domain, comparisons, trends, and gaps were highlighted in both narrative and summary table formats.
Critical appraisal
Although narrative reviews typically omit formal quality weighting, this study assessed methodological rigor:
-
Included only studies providing clear experimental design or validated modeling approaches.
-
Gave preferential weight to pilot-scale work or studies with full life-cycle assessments.
-
Noted and discussed limitations, such as small sample sizes, scalability uncertainty, or high production costs, integrating these critiques within the narrative discussion.
Results and discussion
Algal biorefineries: concept and design
The mass of microalgal strains are suitable producers for biodiesel due to their rich oleaginous contents (50–70%), as shown in Fig. 2. To give an example, the microalga Botryococcus braunii assimilates as far as 80% of its biomass as oil [19, 76, 100]. Auxenochlorella protothecoides and Botryococcus braunii have a high concentration of glyceryl lipids, and terpenoid hydrocarbons that are degradable into small chain hydrocarbons as the main fossil oil [134]. They can convert 9–10% of sunlight-powered energy into staple with an output potential of roughly 280 tons/ha/year, or 77 g/biomass/m2/day [36, 79]. Microalgae can proliferate with a growth rate of 1–3 doublings daily and thrive in various water conditions. In addition, they are unpalatable, so they have no impact on food web [5]. Microalgae are farmable throughout the year in any season, and they contribute to the mitigation of atmospheric CO2 on top of the treatment of wastewater [55, 118]. The lack of lignocellulosic components in microalgal cell walls lowers the overall production costs and streamlines the pre-treatment procedure [93, 117, 142].
Upstream and downstream processes for microalgal biofuel production
Upstreaming and down streaming techniques are regarded as the touchstone in microalgae fieldwork. These operations are indispensable, technically and economically, as they precisely act on the quality and quantity of the developed microalgal biomass, as illustrated in Fig. 3 [25].
Cultivation of algal biomass
A vital component of the synthesis of biofuel is microalgal cultivation. The choice of cultivation system is especially crucial, since it leverages the biofuel turnout and phytoremediation potency [108]. The microalgal cultivation approaches can be broadly catalogued into two major divisions: closed systems (photobioreactors) and open-air systems (ponds) [86]. The largest industrial cultivation methodologies are incorporated in systems that operate in open air [134]. Algae are cultivated in buoyancy with supplementary fertilizers [66, 115]. Cultivation involves natural contact with the soil or using a basic surface covering to reduce seepage-induced water loss [66]. A low-cost, low-energy paddle wheel and pivoted agitator can be used to provide the medium stirring mechanism, which is crucial for maintaining aeration and nutrient dispersion as well as preventing algae settlement [134].
Certain varieties of microalgae might grow in closed systems under highly controlled environments, with conditions of desirable stirring and light availability [65, 72]. PBRs (photobioreactors) preset the mixing and growing conditions. In these closed systems, the water is circulated, whether by pumps or airlift, where the cultures are confined within a transparent recipient, and the algae are grown in suspension [66, 103]. PBRs can be installed indoors and outdoors, under artificial light or sunlight, or both. One of its popular modifications involves the usage of fiber optics to transport sunbeams to a culture installed indoors. The major advantages of the use of PBRs are favorable productiveness, minute spoilation, streamlined CO2 mitigation, incessant performance, and growth condition control, Fig. 3.
Harvesting and drying
The micro size of algae hinders the harvesting step, being dependent upon a number of variables related to cell density and type, the need for subsequent processing, and the merit of the final merchandise given [39]. Biomass pre-treatment may become necessary to enhance the harvesting yield by centrifugation, filtration, sedimentation, ultrasonic aggregation, and even flocculation [30, 115]. Harvesting aims at the slurry production harboring 2–7% suspension of algal cells based on dry matter [115]. Remaining intracellular water in the cells after draining may be removed by a flash dryer, spray dryer, drum dryer, freeze dryer, or thermal drying. Up to 95% dry matter of the biomass is required [71]. During primary or bulk harvesting, a biomass concentrate of 2–7% of total suspended solids is produced using sedimentation, flocculation, and flotation. Next comes a step to thicken the concentrate that uses filtration or centrifugation to create an algal cake with 15–25% TSS; being more resource-draining than initial harvesting [39]. Although many harvesting techniques have come forth in the preceding 40 years, they can be broadly divided into technologies applied in a single or two-step procedure.
Extraction and purification
Systematized material extrication from the inside of the cells requires cell disruption[71]. The techniques of cell disruption could be branched into two divisions: automated ones such as homogenizers, bead mills, etc. and manual methods such as organic solvents and osmotic shock [112]. Following the disruption of cell, numerous oil extraction approaches from microalgae have been implemented, such as presses, supercritical carbon dioxide extraction, ultrasonic-assisted extraction, and more [30, 78]. The first three techniques are only applied in lab settings. The expenditure, effectiveness, noxiousness, and easy handling make up the major crucial factors to consider when choosing the best oil extraction method. Supercritical carbon dioxide and osmotic shock are not commercially feasible because of their high operating costs. Commercializing the enzymatic extraction method is feasible, but cost-reduction measures are required [78].
Techno-economic feasibility
The techno-economic analysis assesses the integrated energetic and financial performance of a processing method. TEA will help analyze how effectively the processing of the crops results in cutting costs [64]. Algal oil is speculated to become one of the major energy feedstocks in years to come. There will not be a complete outline of the research, development, and commercialization of algae-to-fuel technology if the regard of the possible cost of the technology is not taken into consideration [125]. In general, the expense of production of microalgal biofuels is based on a number of variables; these include the output biomass, the amount of oil, the dimension of the production framework, and the overheads of oil extraction from microalgal biomass. The attributes of the resultant biogenic diesel are driven by the oleaginous constitution of the algal oil [78]. Different fatty acid profiles can contain both extremes: elevated content of either saturated FAs or PUFAs. Unfortunately, both PUFAs and saturated FAs guarantee substandard oxidative stability and cold flow properties for the oil [125]. The positive features of algal biodiesel, in fact, are useful when associated with greater manufacturing levels and a well-standardized production process. A study on the generation of biodiesel was conducted by comparing the ATS and ORP processes. The production cost of biodiesel from these methods was estimated at $6.27 and $8.34 biodiesel per gallon. These prices did not give positive economic feasibility [46]. Therefore, processing could be cheaper using algae that naturally excrete lipids or those that have been genetically modified to secrete the lipids from direct photosynthetic conversion [38].
Biofuels from algae
Algal biofuels can be considered highly renewable resources compared to traditional fossil fuels, since algae species generally have very high growth rates and can be grown in various conditions [85]. Production of algal biofuels and bio-products (Fig. 4) are inherent in the following processes: anaerobic digestion, transesterification, thermochemical conversion, and fermentation. These were developed to extract energy-rich compounds from algae, capable of sequestering carbon dioxide more efficiently than conventional biofuel crops [82, 95].
Types of biofuels from algae
Algae have gained significant attention as an imperishable alternative for producing biofuels relying on their fast rate of growth, cultivability to grow on non-farmable land, and potential to capture CO₂ [59]. The key biofuels derived from algae are as follows:
Algal biodiesel
Microalgae are a source of algal biodiesel, deriving from the lipids or fats and oils found in microalgae, by their extrusion and transformation into biodiesel by the means of the transesterification process [92]. The process of extracting crude oil from algae involves the reaction of lipids and an alcohol, usually methanol. A catalyst facilitates this reaction, leading to the formation of fatty acid methyl esters (FAME), which are the chemical compounds found in biodiesel [44]. Algal biodiesel is an inexhaustible and decomposable substitute to petroleum-based diesel that promises to limit greenhouse gas emissions and reduce reliance on fossil fuels [47]. The high lipid content of microalgae makes this organism particularly well-suited for fuel production from autoclaved lipid-rich cells or from cells under stress conditions like nutrient limitation, as previously explored [143]. Their efficiency of lipid accumulation surpasses terrestrial crops, such as soybean or palm oil, which need more land, water, and energy inputs [22]. Algae’s sustainability profile benefits from their potential to propagate in non-arable conditions, such as brackish or waste water. Algae also has the potential to provide substantially higher biodiesel per hectare yields compared to traditional crops that constitute a scalable and sustainable biofuel option [85, 99]. Various species of microalgae have been used for the production of biodiesel by transesterification process, giving different yields, as shown in Table 2. However, several challenges remain in improving the manufacturing and lowering expenses to make algal biodiesel commercially viable on a large scale.
Bioethanol from algal carbohydrates
Some algal species store high quantities of carbohydrates, such as starch and cellulose, which can be utilized for generating bioethanol [132]. The breakdown of polysaccharides occurs during which the agitation of these polysaccharides takes place, allowing for the breakdown into simple mono- or oligosaccharides through enzymatic hydrolysis or acid treatment. These simple sugars are then fermented into ethanol by bacteria or yeast [130]. This bioethanol production technique mimics ethanol derived from crops such as sugarcane or corn and provides the critical advantage of not competing directly with the food resources that otherwise make up a common shortcoming of crop-based biofuels [75]. The capability of algae to grow briskly and acclimate to various habitats, including saline, brackish, and waste water, makes them a highly versatile and, therefore, viable staple to produce bioethanol [129]. Algae can thrive on non-arable land, which helps lessen the pressure that traditional biofuel crops exert on arable land. This growth also reduces the environmental issues linked to conventional biofuel crops [116]. Like other fermentable source sugars, algal species rich in carbohydrates are algal species that have high yields for converting to ethanol [45]. Various species of microalgae have been used for the production of bioethanol by fermentation process giving different yields, as shown in Table 3. While they offer all the advantages, there are still challenges: scaling up production and improving conversion efficiency so the commercial viability of algal bioethanol can be advanced.
Biogas from anaerobic microbial decomposition
The biochemical process of anaerobic digestion (AD) involves micro-organisms that break organic substances without employing oxygen and thus, yielding biogas (methane and carbon dioxide), rendering biomass from algae an ideal feedstock for biogas generation [50]. Algae has a good protein and carbohydrate content, which is attractive for biogas conversion, because anaerobic microbes easily hydrolyze these components [74]. In addition, the resulting biogas shows usage in diverse ways, such as electricity and heat generation, or successfully upgraded to biomethane for use as vehicle fuel [81]. Algae have the potential to become a substrate for anaerobic digestion, which is generally considered an economic and energy-efficient method for the treatment of algae in both waste streams and residual algal biomass from other algal biofuel production. Owing to this integration of processes, this resource optimization is oriented toward a circular bioeconomy by transforming waste into renewable energy [114, 126]. Moreover, algal biomass has been anaerobically digested, and the resulting high-nutrient digestate can be recycled as fertilizer, complementing the sustainability of this biofuel pathway [114]. Various species of microalgae have been used to produce biogas by anaerobic digestion, giving different yields, as shown in Table 4. While the process is efficient, methane yields can be optimized, and system scalability can be improved as the process finds greater commercial application.
Hydrogen production from algae
Photobiological water splitting allows algae (mostly microalgae such as Chlamydomonas reinhartdi) to produce hydrogen gas (H2) using light energy, converting it into hydrogen and oxygen from water [124]. In particular, hydrogenase enzymes in the algae facilitate this process under specific conditions, catalyzing hydrogen production. Algae can be placed in an anaerobic condition, where their metabolic pathway transitions from general photosynthesis to hydrogen production, providing a previously untapped source of renewable, zero-emission energy [32, 33]. You can then use the produced hydrogen to fuel hydrogen-based fuel cells that generate electric power by reacting hydrogen and oxygen that produces just water as a product. Algal hydrogen production is thus a promising approach to sustainable energy [113]. Various species of microalgae have been used to produce biohydrogen, each giving a different yield, as shown in Table 5. However, several challenges prevent the large-scale implementation of this technology, including low hydrogen production efficiency and the technical complexity of maintaining optimal conditions for sustained hydrogenase activity. The yield and scalability of photobiological H2 production must be enhanced as a crucial research area to make it a clean and viable energy supply.
High-value bioproducts from algal biorefineries
Algal biorefineries are designed to convert algal biomass into various products, as shown in Fig. 4, maximizing the use of every component to ensure efficiency and economic viability. High-value bioproducts derived from microalgae are discussed below.
Nutraceuticals
Marine algae are known for being a venerated reservoir of omega-3 fatty acids, predominantly EPA and DHA, as vital nutrients for the human body, i.e., it cannot synthesize them independently [96]. Omega-3 fatty acids that were found in fish oil can now be obtained from algae: while these algae are a fundamental piece of the marine food chain, their existence makes them a sustainable and vegan-friendly alternative [107]. Algae-based cultivation of omega-3 provides an alternative to overfishing the aquatic ecosystems and addresses environmental issues from fish oil production. Besides omega-3 content, algae contain essential vitamins such as A, C, E, and B and essential minerals such as iron and calcium, thereby making them wonderful ingredients in dietary supplements and superfoods [97, 136]. Algal nutraceuticals have been well-researched for their positive health benefits; among others, there is an ability of algal nutraceuticals to reduce total triglyceride and improve lipid profile. Besides, the consumption of omega-3-rich algae is related to the improvement of cognitive functions and neuroprotection, which falls in line with the prevention of risk for neurodegenerative diseases. Algal sources of omega-3 fatty acids are crucial for prevention of obesity and metabolic syndrome [8]. This trend for sustainable nutrition was manifested by health strategies aimed at prevention and dietary supplementation, with a rising interest in algae as one such source of nutraceuticals based on the growing need to use alternative sources for such essential nutrients whose production is ethical and environmentally sound.
Cosmetics (skin care products)
The application of algae as bioactive compounds source was of growing importance for cosmetic industries, as algae gain compounds that assured multiple skincare benefits: anti-aging, moisturizing, and UV protection. It is found that various species of carotenoids, polysaccharides, and protein extracts provide functional properties to support skin health, including Chlorella and Dunaliella, among others [9]. The role of carotenoids is crucial for maintaining a barrier against environmental stressors such as pollution and UV radiation by neutralizing free radicals responsible for oxidative damage. This antioxidant activity not only improves visible signs of aging, such as wrinkles and fine lines, but it also promotes cellular regeneration for an improvement in skin tones and texture [26]. Moreover, algal polysaccharides are highly moisturizing, they develop protective barrier properties on the skin that can be of help in conserving moisture and improving elasticity of the skin. In cosmetic formulations, this hydrating effect is what it mainly can do to help improve hydration of skin and develop visual effects of fuller skin [138]. Besides, algal proteins take part in providing skin structure with firmness and less sag. Because of such multi-functionality, these extracts of algae make a valued active in such cosmetic products as creams, lotions, and serums by responding to the growing demand for natural and effective means against skin problems [98]. Finally, an increase in the integration of algae into cosmetic formulations reflects a greater interest in the use of nature for materials that can meet the needs of sustainability and efficiency in the care of beauty with materials not of synthetic origin.
Animal feed (protein-rich algae meal)
Nowadays, it is being realized that algae are indeed one of the most sustainable and healthy sources of high-quality protein feed for aquaculture, poultry, and livestock farming. Among these, microalgae such as Spirulina and Chlorella are considered very important based on their high-value makeup of vitamins, essential amino acids, and minerals, which are critical during development and health of numerous animal varieties [77]. Nutritional quality is enhanced overall when algal meals are added to animal diets, along with improved growth rates and immune responses that are important for maintaining health and productivity in the animals. Given the reliance on traditional fishmeal, overfishing and environmental degradation is essential in aquaculture [119]. Replacement of fishmeal or soy-based diets with algal protein by the aquaculture industry will go a long way in reducing its ecological footprint in addition to meeting the growing need for sustainable animal feed [49]. Besides, algae are well-cultivated, because the latter can be farmed on agricultural lands unsuitable for other forms crops, for instance, saline or wastewater. This capability frees up land and freshwater resources around competition from structural competition to the agricultural systems and supports food security. In addition, algae can drive carbon sequestration; a reduction in nutrient loading originating from agricultural runoff has further environmental benefits [85]. The incorporation of algae in animal feed is promising from the point of view of improving sustainability in animal husbandry by addressing nutritional needs while mitigating environmental damage.
Bioplastics (PHA, PLA)
Algae-derived bioplastics are increasingly appreciated as a feedstock for biodegradable bioplastics, especially polyhydroxyalkanoates (PHA), and polylactic acid (PLA). PHAs are a family of microbially synthesized polyesters produced by bacteria under nutrient-limited conditions, utilizing sugars, lipids, or waste streams. They exhibit tunable properties from rigid to flexible, and are fully biodegradable in natural environments, including soil, freshwater, and marine settings [94]. In contrast, PLA is a thermoplastic aliphatic polyester derived from plant-based carbohydrates (e.g., corn, sugarcane) via fermentation to lactic acid, followed by polycondensation or ring-opening polymerization; it is industrially compostable under controlled conditions but degrades very slowly in ambient environments [10]. Polyesters synthesized under nutrient-limited growth conditions naturally occur in certain microalgae strains as storage polymers, referred to as PHAs. As such, these bioplastics feature good biodegradability, allowing them to break down naturally in different environments, they are an alternative to conventional petroleum-based plastics that significantly affect environmental pollution [24]. Concomitantly, it is produced by the fermentation of simple sugars from algal carbohydrates. Once the algae polysaccharides are hydrolyzed by enzymes to fermentable sugars, this process usually entails microbial fermentation (i.e., fermentation by bacteria or yeast) to produce lactic acid [88]. PLA is an FDA-approved material used widely in packaging, disposable utensils, and most other applications, because it is made from the polymerization of lactic acid. Algal biomass utilization in the manufacture of bioplastics not only decreases dependence on fossil fuels but also gives a viable environmental solution for waste management as PHAs and PLA are biodegradable and will effectively decrease the plastic waste deposition in landfills and oceans [3]. In addition, algal bioplastics can be generated in different ecological settings, including aquaculture and wastewater treatment facilities, and are part of a circular economy that recycles waste to minimize environmental impact [21]. Algal-based bioplastics development and commercialization potential can fundamentally change the materials industry by addressing global sustainability goals in alignment with the sustainable development of plastic waste and resource depletion.
Environmental and economic benefits
CO₂ mitigation and algae’s role in carbon capture
An essential contribution to carbon sequestration is made by algae primarily because of their unbelievable capacity to photosynthesize appreciable amounts of carbon dioxide [101]. Since algal cultivation exhibits these characteristics, it can be expected to play a vital role as a CO₂ mitigation technology by its capacity to capture CO₂ from industrial emissions, such as power plants and cement factories emissions, or ambient air. Algae usually depend primarily on CO₂ as a source of carbon for photosynthesis with subsequent storage in different biochemical forms, such as carbohydrates, proteins, and lipids. An estimate of the potential of algae to fix carbon of 1.8 tons of CO₂ per ton of dry algal biomass produced has been estimated to show them great potential to be influential biofixers of carbon [20]. Integrating algal cultivation with CCS technology, which refers to a suite of technologies aimed at reducing carbon dioxide (CO₂) emissions from major industrial and power sources by isolating the CO₂, transporting it in compressed form, and permanently storing it in deep geological formations, such as depleted oil and gas fields or saline aquifers [6], also diminishes the emanation of greenhouse gas, facilitating industrial de-carbonization that are challenging to electrify. This coupling improves the lucrative viability of algal biofuel generation using waste CO₂ resources. It aids climate change diminution through direct reduction of the carbon footprint of high emitters like the cement industry [11]. Algae cultivation in this context is thus more than just another energy source; it burgeons from its position as a multi-functional solution to energy and environmental problems and a contributor to the broader sustainability goals. In a climate of conflict with energy and the environment, algae provide new insight into how societies can contribute to an added resilient, carbon less future through the wise wielding of natural reservoirs while furthering economic growth.
Reduced water and land footprint
Compared to conventional biofuel feedstocks, such as corn, soy, and palm oil, algal biorefineries show one of the lowest environmental footprints on land and water. The ability to cultivate algae in saline or brackish water, including wastewater, besides growing it in non-arable land, represents an added ability to grow it without competing with more traditional agricultural resources [85]. This is particularly important considering global decreases in available water and degraded land, as this can be accomplished without fresh water and fertile soil crucial to terrestrial crops. Different algal systems open ponds to photobioreactors, and closed-loop systems using wastewater as a growth medium make use of underutilized resources that contribute to greater efficiency [102]. Wastewater can act as a great supply of nutrients for algae growth, while it serves the twin purpose of treatment and remediation of wastewater [12, 69]. Its growth under variant conditions enables industrial scale generation of biofuels and bioproducts without arable land depletion or competition for previously limited freshwater supplies, positioning algae as the prime candidate in sustainable agricultural development [11]. These specific features of algae growth allow algal biorefinery to off-balance food security with environmental conservation, according to global goals on sustainability for transitioning toward a more resource-efficient, resilient economy. Including algal production in the presently existing agricultural systems and technologies makes the approach to some mega challenges, climate change, resource depletion, and food production completely new.
Life-cycle analysis of algal biofuels and bioproducts
LCA is a prime tool for reviewing the environmental sustainability of algal biofuels by analyzing the complete life cycle of those biofuels from cultivation to end-of-life disposal. The overall approach indicates that, under ideal situations, algal biofuels would reduce GHG emissions significantly over conventional fossil fuels [141]. As a result, this reduction, of course, entails the use and application of waste CO₂ and non-fresh sources of water, which reduce the environmental burden on the extraction of resources [141]. Moreover, in a consolidated biorefinery, the coproduction of premium co-products such as bioplastics or animal feed will further increase the EROI for algal biofuels [18]. Algal biofuels are sustainable only if the demand of energy for algae cultivation, harvesting, and refinement, as well as the efficiency of the conversion technologies used, are considered [1]. The incorporation of circular-economy strategies and waste-to-energy approaches will further minimize the carbon footprint and the environmental load of algal biofuels and thus enhance their role in sustainable energy systems.
Economic impact
Potential for job creation and rural development
Algal biorefineries also stimulate economic development, especially in agriculture, biotechnology, engineering, and research, hence giving opportunities for jobs within rural or less developed economies [111]. Algal culture, since it is mostly over non-arable lands or saline water, provides a good avenue for earnings to locals and simultaneously reduces rural–urban migrations in search of jobs, thus helping to create a socio-economic equilibrium within these geographical zones [128]. Because of this reason, various regions can cultivate algae in various scales and follow economic development models that are suitable for them so that productive inclusive economic growth can be attained as well as sustained. On the contrary, the algal biotechnology industry motivates educational laborers in the form of artists, engineers as well as scientists, which raises economic productivity. Among other factors, this helps boost the output of the economy [34]. This expansion accordingly brings local jobs and regional competencies that seem to assist algal biorefineries in their supportive role in economic and technological development.
Impact on energy security and scaling down the dependence on fossil fuels
Algal biofuels are a prospective renewable replacement to fossil fuels, and they would drive the world to greater energy security by removing the need for oil and gas from overseas. In producing various biofuels, such as biodiesel, bioethanol, and biogas, algae could add an alternative energy portfolio that mitigates the economic volatility and market risk associated with fossil fuels [51]. Algae are tolerant of many types of climates. They can be grown on the native ground, lowering a country’s dependency on imported energy and creating greater energy independence. Such domestic possibilities are exciting when the world tries to shift to cleaner energy networks [23]. Algal biofuels could also help in other areas, where electrification has yet to happen, such as aviation, marine shipping, and heavy transport, since they put forward a substitute to liquid fuels that are viable and decarbonized [37]. In addition, algal biofuel production aligns with international climate goals, as it reduces greenhouse gas emissions and is vital to achieving renewable energy goals and long-term plans for mitigating climate change.
Challenges and constraints in algal biorefinery development
Despite the transformative promise of algal biorefineries, their progression from laboratory to commercial implementation remains hindered by a constellation of inter-related challenges. A critical, pipeline-oriented analysis—spanning upstream cultivation through downstream processing and culminating in system-level integration—reveals both the severity of these obstacles and the limited scope of current mitigation strategies.
Upstream challenges: cultivation and strain engineering
Insufficient biomass productivity and economic implications:
Although microalgae exhibit rapid doubling times under optimal laboratory conditions, real-world autotrophic cultivation systems rarely surpass 2–3 g L⁻1 biomass concentration. This gap translates into inflated capital expenditure (CAPEX) for reactor infrastructure and elevated operational expenditure (OPEX) for CO₂ and nutrient supplementation. When benchmarked against heterotrophic bacterial fermentations (30–100 g L⁻1), the algal systems’ low volumetric productivity undermines economies of scale, rendering the cost-per-kilogram of biomass an order of magnitude higher [106]. Consequently, without breakthrough improvements in strain or hybrid cultivation modes, the intrinsic economics of algal cultivation remain uncompetitive.
Photobioreactor (PBR) versus open-pond trade-off analysis:
Photobioreactors deliver controlled illumination, resistance to contamination, and consistent yields, yet their CAPEX can exceed that of open-pond systems by 200–300%. Even after accounting for a 2–3 × enhancement in areal productivity, the internal rate of return (IRR) for PBR projects remains marginal under prevailing energy and carbon credit prices. Conversely, open ponds benefit from low initial investment but suffer yield fluctuations due to evaporative losses, predation, and climatic variability [115, 134]. Neither system currently achieves the performance–cost balance required for scaled biofuel production, highlighting a critical need for cost-effective hybrid designs or modular, adaptable photobioreactor architectures.
Genetic and metabolic engineering:
Promise versus Practicality CRISPR/Cas9 and TALENs have unlocked precise pathway manipulations, enhancing lipid titres by up to 30–50% in model strains [53]. However, these gains often come at the expense of trade-offs in growth rate, cellular robustness, and regulatory compliance. Moreover, most engineered microalgae remain at technology readiness levels (TRL) of 3–4, with limited progression to pilot-scale evaluation. The absence of scalable transformation protocols for non-model species and the high cost of selection markers further constrain widespread adoption, underscoring a disconnect between laboratory breakthroughs and industrial application [61, 68].
Midstream challenges: harvesting and dewatering
Excessive energy demand in biomass recovery:
Harvesting microalgal biomass accounts for up to 25–30% of the total process energy consumption. While centrifugation achieves a recovery efficiency of ≥ 90%, it requires 0.8–1.2 kWh m⁻3, contributing directly to both OPEX and embodied energy. Alternative flocculation agents reduce energy inputs but introduce chemical contaminants, which can hinder downstream bioproduct purity and complicate solvent recycling [30, 39]. The field lacks a universally scalable, low-energy harvesting technology, with pilot innovations often retreating to bench-scale due to fouling, variable water chemistries, and regulatory barriers on flocculant residues.
High-cost dewatering and drying processes:
Post-harvest dewatering and drying elevate capital and operational costs significantly. Spray and drum drying techniques can achieve > 95% solids but demand > 3 MJ kg⁻1 of thermal energy, equating to 0.8–1.0 kWh kg⁻1; freeze–drying remains cost-prohibitive and slow. Despite innovations in solar-drying and low-temperature vacuum systems, meteorological dependencies and long residence times limit throughput. The industry currently lacks a validated, large-scale dewatering solution that balances energy consumption, throughput, and biomass integrity [70, 71].
Downstream challenges: extraction and conversion
Scale-up limitations of cell disruption techniques:
Mechanical disruption (bead milling, homogenization) is the only method scalable to industrial volumes but incurs specific energy inputs exceeding 1 kWh kg⁻1 of biomass. Solvent-based extraction, including supercritical CO₂, offers high lipid purity yet is beset by high CAPEX (> USD 5 million pilot units) and limited throughput [78, 112]. Despite decades of research, no extraction protocol has been developed that simultaneously ensures commercial-scale throughput, product purity, and cost-effectiveness.
Multi-product fractionation: process interdependency and cost escalation
The integrated recovery of lipids, proteins, and carbohydrates demands sequential unit operations, each optimized for a specific biomolecule. These interdependent steps magnify CAPEX, as equipment must withstand diverse physico-chemical regimes, and OPEX, due to solvent and enzyme consumption. For example, conditions that favor enzymatic saccharification can denature algal proteins, whereas harsh solvents compromise lipid integrity [18, 54]. The absence of modular, cascade-compatible fractionation platforms exacerbates this complexity, impeding economic viability beyond single-product biorefinery models.
System-level challenges
Techno-economic feasibility deficits
Published techno-economic analyses consistently estimate algal biodiesel production costs in the range of USD 6–8 per gallon, compared to a benchmark of USD 2.50 per gallon for petroleum diesel. Key drivers include nutrient input costs, energy-intensive dewatering, and suboptimal biomass yields [46]. Sensitivity analyses reveal that even a 50% reduction in dewatering energy or a doubling of biomass productivity fails to shift the levelized cost of fuel (LCOF) into a competitive range without substantial policy incentives or carbon pricing mechanisms [125].
Inconsistent environmental benefits and LCA uncertainties
Life-cycle assessments demonstrate potential greenhouse gas emissions reductions exceeding 50% only when non-potable water sources and flue-gas CO₂ are utilized. However, heterogeneity in boundary definitions, functional unit allocation, and sensitivity to co-product credits produces LCA outcomes that vary by ± 30–40% [1, 22]. This variability undermines stakeholder confidence in environmental claims and complicates the formulation of uniform policy incentives.
Integration barriers and regulatory gaps
Coupling algal cultivation with wastewater treatment can substantially offset nutrient costs and deliver effluent polishing benefits. However, fluctuations in influent composition, pathogen risks, and stringent discharge standards demand robust monitoring and contingency strategies. Furthermore, regulatory frameworks for algal-derived co-products (e.g., feed, nutraceuticals) remain fragmented across jurisdictions, which delays market entry and discourages investment [42, 137].
Advancing horizons and strategic research in algal biorefineries
Genetic engineering and synthetic biology
Specific biomolecule synthesis may be aided by adding or deleting specific genes[90]. This procedure can be easily carried out with the microalgae’s genome knowledge. The first algae whose genome was first sequenced was Chlamydomonas reinhardtii [80]. This organism serves as a prototype organism for the development of numerous tools. Numerous varieties have already been sequenced using NGS technologies. Agrobacterium-mediated transformations, glass beads, biolistic, and electroporation are methods developed to introduce foreign DNA into microalgae [67]. Tools for chloroplast transformation have been created to augment the expression of a particular gene [89]. In addition, researchers created directed gene editing in microalgae. Scar-free gene editing has been made possible by the use of CRISPR–Cas9, which allows for the deletion of particular genes, the improvement of a phenotype, or the blocking of competitive pathways to increase the synthesis of particular compounds [84, 87]. Even with recent developments, genetic editing is still lacking in algae compared to bacteria and fungi. Commercialization of the algae’s bioproducts has been attempted, but few researchers are interested due to limitations [29].
Advances in genome editing techniques for enhancing microalgal biomass and lipid composition
Methodologies for genome editing improve the caliber and yield of microalgae goods. These techniques include, transcription activator-like effector nucleases (TALENs), CRISPR/Cas9, RNA interference (RNAi) and zinc finger nucleases (ZFNs). RNA interference serves as an effective technique for augmenting the microalgal portion of lipid, specifically in Phaeodactylum tricornutum, by targeting the gene involved in the expression of nitrate reductase. The process of gene silencing has been successfully implemented, leading to a significant enhancement of the metabolic pathways associated with the biosynthesis of lipid [68].
ZFNs (Zinc Finger Nucleases) and TALENs (Transcription Activator-Like Effector Nucleases) function by cleaving the specific DNA target sequence through the action of the FokI nuclease, which is directed by protein elements. Genome editing methodologies are increasingly adopted, yet their implementation in microalgae remains relatively limited. Although genetic modification has the potential to enhance lipid accumulation, it does not invariably result in elevated biomass productivity. The strategy of suppressing starch biosynthesis to augment lipid content may pose certain risks, as it can lead to a decline in the generation of biomass, which is vital for the synthesis of biofuel [139]. To enhance lipid production, platinum TALENs are used to focus on the acyltransferase gene (LPAT1) in Nannochloropsis oceanica and the nitrate reductase gene (NoNR) [61].
The most effective technique is CRISPR/Cas9, which has attracted considerable interest owing to its precision and simplicity. This approach employs RNA-guided Cas9 nuclease to generate precise breaks in DNA. Thus far, it has been utilized across various microalgal strains, including Phaeodactylum tricornutum, Chlorella species, Nannochloropsis species, and Chlamydomonas reinhardtii [28]. For instance, the disruption of the AATPL1 gene resulted in a 30% enhancement in the oleaginous composition of Parachlorella kessleri in comparison with wild varieties [53, 53]. Moreover, utilizing DNA-free CRISPR/Cas9 ribonucleoprotein (RNP) methodologies, the lipid content in Tetraselmis sp. was elevated by as much as 3.1-fold during conditions of nitrogen deprivation, in contrast to the wild-type strain. (7.68% of DCW) [15].
Automation and AI in algal biorefineries
Therefore, the role of AI, which can feed information on high uncertainty biosystems, is developing daily in microalgal research. In AI, the algorithms can be divided into the following three fields: expert systems, machine learning, and metaheuristics. An advanced machine learning framework like active learning reduces the number of experiments. It gives efficient sampling techniques in experiments on microalgae [31]. Another subcategory of ML effective in research on microalgae is meta-learning. The meta-learning theory is properly defined; through this approach, ML tasks are automated or sped up using metadata [133]. It was studied that RNA-seq meta-analysis in microalgae can be done by meta-learning, unveiling critical biological pathways [91]. It is easy to envisage that neuro-evolution-based meta-learning will be able to predict the optimization of the thermal transformation of microalgae [122]. Besides, a number of meta-learning methods have been attentively applied to predict algae growth—a field that is opening more and more vistas for its application in the future [110]. Semi-supervised learning, better known as generative models, is developing a load of applications for designing sequences and editing genomes so as to produce automatically desirable genome sequences [73].
IoT technologies and algal biorefinery
Algal biorefinery is eco-friendly, but it still demands technical enhancement. Inventive implementations of Internet of Things (IoT) techniques can fulfill its demands. These include productivity optimization, toxic species recognition, automated self-regulated algae cultivation variables of administration and manipulation, screening specific algal species, algal cell sustainability detection, and microalgae species classification. IoT techniques are essential to achieve intelligent, economic, automated, and green technology for microalgae biorefinery [123].
Integrating algal biorefinery with wastewater treatment
Wastewater treatment by microalgae is more effective, because microalgae use CO2 and pollutants, have a rapid growth rate, and can be grown on any non-agricultural land [42, 62]. CO2 is essential in fatty acid production and algae growth [19]. So, integrating wastewater treatment and biofuel production can have the best outcome for biofuel and power generation. It also has excellent environmental and economic benefits if we combine both processes. Many studies showed that different microalgae species could be cultivated in different types of wastewaters, decreasing the biofuel production cost [42]. They can be mass-produced and provide different nutrients [144].
More research is advised on a few topics to make it more cost-effective and feasible for synthesis on an industrial scale, such as improving algal biorefinery technologies and increasing the amount of algal biofuel produced using wastewater. Innovative research is necessary for the commercialization of algal biorefineries. The limitations of the process can be assessed with the aid of LCA and TEA, which will stimulate additional research. The future will see the formulation of new government policies [137].
Conclusion
The present study examines how algal biorefineries have the capacity to disrupt the current energy and environmental sustainability paradigm. The algal biorefinery concept represents a compelling, sustainable platform for biofuel and bioproduct production, but several obstacles threaten its large-scale implementation. Algal high-rate growth at higher lipid levels on non-arable lands and wastewater makes it the perfect alternative to current biofuel feedstocks. Moreover, the blending of the algal biorefinery concept with the rules regimental to a circular economy will contribute more toward its sustainability as well, because it not only makes the most of recycling to ensure optimal efficiency of a resource but also minimizes waste production in forms. Nonetheless, this study pinpoints several bottlenecks which might be counter-productive in the future. Some key barriers include high operational costs due to the usage of expensive inputs, including nutrients and CO2, and high energy demand during the cultivation stage or upstream processing. Low energetic conversion efficiency between input and output streams is facilitated by downstream system inefficiencies, collectively posing questions on their economic feasibility. In addition, the techno-economic analyses reveal that current algal biofuel production costs remain uncompetitive with fossil fuels, necessitating further optimization and policy intervention. This review underscores the importance of integrating algal biorefineries with systems such as wastewater treatment to offset costs and provide environmental co-benefits. Ultimately, while algal biorefineries hold transformative potential, their realization as a cornerstone of the global bioeconomy depends on overcoming significant technological and economic hurdles through sustained research, innovation, and supportive regulatory frameworks.
Data availability
No data sets were generated or analyzed during the current study.
References
-
Abdelaziz AEM, Leite GB, Hallenbeck PC. Addressing the challenges for sustainable production of algal biofuels: II harvesting and conversion to biofuels. Environ Technol. 2013;34:1807–36. https://doi.org/10.1080/09593330.2013.831487.
-
Abdur Razzak S, Bahar K, Islam KM, Haniffa A, Faruque MO, Hossain SM, Hossain M. Microalgae cultivation in photobioreactors: sustainable solutions for a greener future. Green Chem Engin. 2023. https://doi.org/10.1016/j.gce.2023.10.004.
-
Adeleye AT, Odoh CK, Enudi OC, Banjoko OO, Osiboye OO, Toluwalope Odediran E, Louis H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process Biochem. 2020;96:174–93. https://doi.org/10.1016/j.procbio.2020.05.032.
-
Ahmad S, Shukla AK, Kumar A. How to write the scientific research paper-a review study. Int J Contemp Pathol. 2016;2(1):69. https://doi.org/10.5958/2395-1184.2016.00017.6.
-
Aljabri H, Das P, Khan S, Thaher M, Abdulquadir M. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—a review. Water. 2020. https://doi.org/10.3390/w13010027.
-
Anderson S, Newell R. Prospects for carbon capture and storage technologies. Annu Rev Environ Res. 2004;29(1):109–42. https://doi.org/10.1146/annurev.energy.29.082703.145619.
-
Augusta M, Gasparotto GA, Goldbeck R. Enzymatic hydrolysis of carbohydrate-rich Chlorella vulgaris for third-generation bioethanol production by cellulase-recombinant yeast. Clean Chem Engin. 2023;6:100111–100111. https://doi.org/10.1016/j.clce.2023.100111.
-
Avallone R, Vitale G, Bertolotti M. Omega-3 fatty acids and neurodegenerative diseases: new evidence in clinical trials. Int J Mol Sci. 2019;20: 4256. https://doi.org/10.3390/ijms20174256.
-
Baky HHAE, El-Baroty GS. Healthy benefit of microalgal bioactive substances. J Aquatic Sci. 2013;1:11–22. https://doi.org/10.12691/jas-1-1-3.
-
Balla E, Daniilidis V, Karlioti G, Kalamas T, Stefanidou M, Bikiaris ND, Vlachopoulos A, Koumentakou I, Bikiaris DN. Poly(lactic acid): a versatile biobased polymer for the future with multifunctional properties—from monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers. 2021;13(11):1822. https://doi.org/10.3390/polym13111822.
-
Beal CM, Archibald I, Huntley ME, Greene CH, Johnson ZI. Integrating algae with bioenergy carbon capture and storage (ABECCS) increases sustainability. Earths Future. 2018;6:524–42. https://doi.org/10.1002/2017ef000704.
-
Bhandari M, Kumar P, Bhatt P, Simsek H, Kumar R, Chaudhary A, Malik A, Prajapati SK. An integration of algae-mediated wastewater treatment and resource recovery through anaerobic digestion. J Environ Manag. 2023;342: 118159. https://doi.org/10.1016/j.jenvman.2023.118159.
-
Brennan L, Owende P. Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev. 2009;14(2):557–77. https://doi.org/10.1016/j.rser.2009.10.009.
-
Caporgno MP, Clavero E, Torras C, Salvadó J, Lepine O, Pruvost J, Legrand J, Giralt J, Bengoa C. Energy and nutrients recovery from lipidextracted Nannochloropsis via anaerobic digestion and hydrothermal liquefaction. ACS Sustain Chem Eng. 2016;4(6):3133–9. https://doi.org/10.1021/acssuschemeng.6b00151.
-
Chang KS, Kim J, Park H, Hong S-J, Lee C-G, Jin E. Enhanced lipid productivity in AGP knockout marine microalga Tetraselmis sp. using a DNA-free CRISPR-Cas9 RNP method. Bioresour Technol. 2020;303: 122932. https://doi.org/10.1016/j.biortech.2020.122932.
-
Chen J, Li Y, Li M, Shi J, Wang L, Luo S, Liu H. Chemical flocculation-based green algae materials for photobiological hydrogen production. ACS Appl Bio Mater. 2022;5(2):897–903. https://doi.org/10.1021/acsabm.1c01281.
-
Chen Y, Xu C, Vaidyanathan S. Microalgae: a robust “green bio-bridge” between energy and environment. Crit Rev Biotechnol. 2017;38(3):351–68. https://doi.org/10.1080/07388551.2017.1355774.
-
Chew KW, Yap JY, Show PL, Suan NH, Juan JC, Ling TC, Lee D-J, Chang J-S. Microalgae biorefinery: High value products perspectives. Bioresour Technol. 2017;229:53–62. https://doi.org/10.1016/j.biortech.2017.01.006.
-
Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25(3):294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001.
-
Choi HI, Sung YJ, Hong ME, Han J, Min BK, Sim SJ. Reconsidering the potential of direct microalgal biomass utilization as end-products: a review. Renew Sustain Energ Rev. 2022;155:111930–111930. https://doi.org/10.1016/j.rser.2021.111930.
-
Chong JWR, Khoo KS, Yew GY, Leong WH, Lim JW, Lam MK, Ho Y-C, Ng HS, Munawaroh HSH, Show PL. Advances in production of bioplastics by microalgae using food waste hydrolysate and wastewater: a review. Bioresour Technol. 2021;342: 125947. https://doi.org/10.1016/j.biortech.2021.125947.
-
Clarens AF, Resurreccion EP, White MA, Colosi LM. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol. 2010;44:1813–9. https://doi.org/10.1021/es902838n.
-
Correa DF, Beyer HL, Possingham HP, Fargione JE, Hill JD, Schenk PM. Microalgal biofuel production at national scales: reducing conflicts with agricultural lands and biodiversity within countries. Energy. 2020;215: 119033. https://doi.org/10.1016/j.energy.2020.119033.
-
Costa SS, Miranda AL, de Morais MG, Costa JAV, Druzian JI. Microalgae as source of polyhydroxyalkanoates (PHAs) — a review. Int J Biol Macromol. 2019;131:536–47. https://doi.org/10.1016/j.ijbiomac.2019.03.099.
-
Daneshvar E, Ok YS, Tavakoli S, Sarkar B, Shaheen S, Hong 洪惠 H, Luo Y, Rinklebe J, Song H, Bhatnagar A. Insights into upstream processing of microalgae: a review. Bioresour Technol. 2021; 329: 124870. https://doi.org/10.1016/j.biortech.2021.124870
-
Darvin ME, Sterry W, Lademann J, Vergou T. The role of carotenoids in human skin. Molecules. 2011;16:10491–506. https://doi.org/10.3390/molecules161210491.
-
de Jesus SS, Ferreira GF, Moreira LS, Filho RM. Biodiesel production from microalgae by direct transesterification using green solvents. Renew Energ. 2020;160:1283–94. https://doi.org/10.1016/j.renene.2020.07.056.
-
Dhokane D, Shaikh A, Yadav A, Giri N, Bandyopadhyay A, Dasgupta S, Bhadra B. CRISPR-based bioengineering in microalgae for production of industrially important biomolecules. Front Bioengin Biotechnol. 2023. https://doi.org/10.3389/fbioe.2023.1267826.
-
Di Visconte GS, Spicer A, Chuck CJ, Allen MJ. The microalgae biorefinery: a perspective on the current status and future opportunities using Genetic modification. Appl Sci. 2019;9(22):4793. https://doi.org/10.3390/app9224793.
-
Dragone G, Fernandes B, Vicente A, Teixeira J. Third generation biofuels from microalgae, vol. 2. Badajoz: Formatex Research Center; 2010. p. 1355–66.
-
Drews P, Colares RG, Machado P, De Faria M, Detoni A, Tavano V. Microalgae classification using semi-supervised and active learning based on Gaussian mixture models. J Braz Comput Soc. 2013;19(4):411–22. https://doi.org/10.1007/s13173-013-0121-y.
-
Dubini A, Ghirardi ML. Engineering photosynthetic organisms for the production of biohydrogen. Photosynth Res. 2014;123:241–53. https://doi.org/10.1007/s11120-014-9991-x.
-
Eckert C, Dubini A, Yu J, King P, Ghirardi M, Seibert M, Maness PC. Hydrogenase genes and enzymes involved in solar hydrogen production. State Art Prog Prod Biohydrogen. 2012. https://doi.org/10.2174/978160805224011201010008.
-
Fabris M, Abbriano RM, Pernice M, Sutherland DL, Commault AS, Hall CC, Labeeuw L, McCauley JI, Kuzhiuparambil U, Ray P, Kahlke T, Ralph PJ. Emerging technologies in algal biotechnology: toward the establishment of a sustainable, algae-based bioeconomy [Review]. Front Plant Sci. 2020. https://doi.org/10.3389/fpls.2020.00279.
-
Fernando S, Adhikari S, Chandrapal C, Murali N. Biorefineries: current status, challenges, and future direction. Energy Fuels. 2006;20(4):1727–37. https://doi.org/10.1021/ef060097w.
-
Formighieri C, Franck F, Bassi R. Regulation of the pigment optical density of an algal cell: filling the gap between photosynthetic productivity in the laboratory and in mass culture. J Biotechnol. 2012;162(1):115–23. https://doi.org/10.1016/j.jbiotec.2012.02.021.
-
Fragkos P. Decarbonizing the international shipping and aviation sectors. Energies. 2022. https://doi.org/10.3390/en15249650.
-
Georgianna DR, Mayfield SP. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature. 2012;488(7411):329–35. https://doi.org/10.1038/nature11479.
-
Gerardo ML, Van Den Hende S, Vervaeren H, Coward T, Skill SC. Harvesting of microalgae within a biorefinery approach: a review of the developments and case studies from pilot-plants. Algal Res. 2015;11:248–62. https://doi.org/10.1016/j.algal.2015.06.019.
-
Goli A, Shamiri A, Talaiekhozani A, Eshtiaghi N, Aghamohammadi N, Aroua MK. An overview of biological processes and their potential for CO 2 capture. J Environ Manage. 2016;183:41–58. https://doi.org/10.1016/j.jenvman.2016.08.054.
-
González-González LM, Zhou L, Astals S, Thomas-Hall SR, Eltanahy E, Pratt S, Jensen PD, Schenk PM. Biogas production coupled to repeat microalgae cultivation using a closed nutrient loop. Bioresour Technol. 2018;263:625–30. https://doi.org/10.1016/j.biortech.2018.05.039.
-
Goswami RK, Agrawal K, Mehariya S, Molino A, Musmarra D, Verma P. Microalgae-based biorefinery for utilization of carbon dioxide for production of valuable bioproducts. In: Chemo-biological systems for CO2 utilization. Boca Raton: CRC Press; 2020.
-
Hariskos I, Posten C. Biorefinery of microalgae – opportunities and constraints for different production scenarios. Biotechnol J. 2014;9(6):739–52. https://doi.org/10.1002/biot.201300142.
-
Hidalgo P, Ciudad G, Schober S, Mittelbach M, Navia R. Biodiesel synthesis by direct transesterification of microalga Botryococcus braunii with continuous methanol reflux. Bioresour Technol. 2015;181:32–9. https://doi.org/10.1016/j.biortech.2015.01.047.
-
Ho S-H, Huang S-W, Chen C-Y, Hasunuma T, Kondo A, Chang J-S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour Technol. 2013;135:191–8. https://doi.org/10.1016/j.biortech.2012.10.015.
-
Hoffman J, Pate RC, Drennen T, Quinn JC. Techno-economic assessment of open microalgae production systems. Algal Res. 2017;23:51–7. https://doi.org/10.1016/j.algal.2017.01.005.
-
Hundt K, Reddy BV. Algal biodiesel production from power plant exhaust and its potential to replace petrodiesel and reduce greenhouse gas emissions. Int J Low-Carbon Technol. 2011;6:294–8. https://doi.org/10.1093/ijlct/ctr017.
-
Hupp B, Pap B, Farkas A, Maróti G. Development of a microalgae-based continuous starch-to-hydrogen conversion approach. Fermentation. 2022. https://doi.org/10.3390/fermentation8070294.
-
Idenyi JN, Eya JC, Nwankwegu AS, Nwoba EG. Aquaculture sustainability through alternative dietary ingredients: microalgal value-added products. Engin Microbiol. 2022;2: 100049. https://doi.org/10.1016/j.engmic.2022.100049.
-
Jameel MK, Mustafa MA, Ahmed HS, Mohammed AJ, Ghazy H, Shakir MN, Lawas AM, Mohammed SK, Idan AH, Mahmoud ZH, Sayadi H, Kianfar E. Biogas: production, properties, applications, economic and challenges: a review. Results Chem. 2024;7: 101549. https://doi.org/10.1016/j.rechem.2024.101549.
-
Jones C. Algae biofuels: versatility for the future of bioenergy. Curr Opin Biotechnol. 2011;23:346–51. https://doi.org/10.1016/j.copbio.2011.10.013.
-
Kannah RY, Kavitha S, Banu JR, Sivashanmugam P, Gunasekaran M, Kumar G. A mini review of biochemical conversion of algal biorefinery. Energy Fuels. 2021;35(21):16995–7007. https://doi.org/10.1021/acs.energyfuels.1c02294.
-
Kasai Y, Takagi S, Ota S, Ishii K, Takeshita T, Kawano S, Harayama S. Development of a CRISPR/Cas9-mediated gene-editing method to isolate a mutant of the unicellular green alga Parachlorella kessleri strain NIES-2152 with improved lipid productivity. Biotechnol Biofuels Bioprod. 2024. https://doi.org/10.1186/s13068-024-02484-7.
-
Katiyar R, Banerjee S, Arora A. Recent advances in the integrated biorefinery concept for the valorization of algal biomass through sustainable routes. Biofuels Bioprod Biorefin. 2021. https://doi.org/10.1002/bbb.2187.
-
Khan MI, Shin JH, Kim JD. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact. 2018. https://doi.org/10.1186/s12934-018-0879-x.
-
Kim HM, Wi SG, Jung S, Song Y, Bae H-J. Efficient approach for bioethanol production from red seaweed Gelidium amansii. Bioresour Technol. 2015;175:128–34. https://doi.org/10.1016/j.biortech.2014.10.050.
-
Kim KH, Lee OK, Kim CH, Seo J-W, Oh B-R, Lee EY. Lipase-catalyzed in-situ biosynthesis of glycerol-free biodiesel from heterotrophic microalgae, Aurantiochytrium sp. KRS101 biomass. Bioresour Technol. 2016;211:472–7. https://doi.org/10.1016/j.biortech.2016.03.092.
-
Kisielewska M, Dębowski M, Zieliński M. Comparison of biogas production from anaerobic digestion of microalgae species belonged to various taxonomic groups. Arch Environ Prot. 2020;46(1):33–40. https://doi.org/10.24425/aep.2020.132523.
-
Klinthong W, Yang Y-H, Huang C-H, Tan C-S. A review: microalgae and their applications in CO2 capture and renewable energy. Aerosol Air Qual Res. 2015;15:712–42. https://doi.org/10.4209/aaqr.2014.11.0299.
-
Kumar D, Singh B. Algal biorefinery: an integrated approach for sustainable biodiesel production. Biomass Bioenerg. 2019;131: 105398. https://doi.org/10.1016/j.biombioe.2019.105398.
-
Kurita T, Moroi K, Iwai M, Okazaki K, Shimizu S, Nomura S, Saito F, Maeda S, Takami A, Sakamoto A, Ohta H, Sakuma T, Yamamoto T. Efficient and multiplexable genome editing using Platinum TALENs in oleaginous microalga, Nannochloropsis oceanica NIES-2145. Genes Cells. 2020;25(10):695–702. https://doi.org/10.1111/gtc.12805.
-
Lam MK, Lee KT, Mohamed AR. Current status and challenges on microalgae-based carbon capture. Int J Greenhouse Gas Control. 2012;10:456–69. https://doi.org/10.1016/j.ijggc.2012.07.010.
-
Lamb JJ, Hjelme DR, Lien KM. Carbohydrate yield and biomethane potential from enzymatically hydrolysed Saccharina latissima and its industrial potential. Adv Microbiol. 2019;09(04):359–71. https://doi.org/10.4236/aim.2019.94021.
-
Laurens LML, Slaby EF, Clapper GM, Howell S, Scott D. Algal biomass for biofuels and bioproducts: overview of boundary conditions and regulatory landscape to define future algal biorefineries. Ind Biotechnol. 2015;11(4):221–8. https://doi.org/10.1089/ind.2015.0007.
-
Lee OK, Lee EY. Sustainable production of bioethanol from renewable brown algae biomass. Biomass Bioenergy. 2016;92:70–5. https://doi.org/10.1016/j.biombioe.2016.03.038.
-
Leite GB, Abdelaziz AEM, Hallenbeck PC. Algal biofuels: Challenges and opportunities. Bioresour Technol. 2013;145:134–41. https://doi.org/10.1016/j.biortech.2013.02.007.
-
León R, Fernández E. Nuclear transformation of eukaryotic microalgae. In: León R, Fernández E, editors. Advances in experimental medicine and biology. Berlin: Springer; 2008. p. 1–11.
-
Levitan O, Dinamarca J, Zelzion E, Gorbunov MY, Falkowski PG. Anrnainterference knock-down of nitrate reductase enhances lipid biosynthesis in the diatomphaeodactylum tricornutum. Plant J. 2015;84(5):963–73. https://doi.org/10.1111/tpj.13052.
-
Li Y, Tarpeh WA, Nelson KL, Strathmann TJ. Quantitative evaluation of an integrated system for valorization of wastewater algae as bio-oil, fuel gas, and fertilizer products. Environ Sci Technol. 2018;52:12717–27. https://doi.org/10.1021/acs.est.8b04035.
-
Liang MH, Zhu J, Jiang JG. High-value bioproducts from microalgae: Strategies and progress. Crit Rev Food Sci Nutr. 2018;59:01–53. https://doi.org/10.1080/10408398.2018.1455030.
-
Liang Y, Kashdan T, Sterner C, Dombrowski L, Petrick I, Kröger M, Höfer R. Algal biorefineries. In: Liang Y, Kashdan T, Sterner C, editors. Industrial biorefineries & white biotechnology. Amsterdam: Elsevier; 2015. p. 35–90.
-
Liao Q, Chang JS, Herrmann C, Xia A. Bioreactors for microbial biomass and energy conversion. Berlin/Heidelberg: Springer; 2018.
-
Linder J, Bogard N, Rosenberg AB, Seelig G. A generative neural network for maximizing fitness and diversity of synthetic DNA and protein sequences. Cell Syst. 2020;11(1):49-62.e16. https://doi.org/10.1016/j.cels.2020.05.007.
-
Magdalena J, Ballesteros M, González-Fernandez C. Efficient anaerobic digestion of microalgae biomass: proteins as a key macromolecule. Molecules. 2018;23:1098. https://doi.org/10.3390/molecules23051098.
-
Malode S. Recent advances and viability in biofuel production. Energ Convers Manag X. 2020;10: 100070. https://doi.org/10.1016/j.ecmx.2020.100070.
-
Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sustain Energ Rev. 2009;14(1):217–32. https://doi.org/10.1016/j.rser.2009.07.020.
-
Matos J, Cardoso C, Bandarra NM, Afonso C. Microalgae as healthy ingredients for functional food: a review. Food Funct. 2017;8:2672–85. https://doi.org/10.1039/c7fo00409e.
-
Medipally SR, Yusoff FM, Banerjee S, Shariff M. Microalgae as sustainable renewable energy feedstock for biofuel production. BioMed Res Int. 2015;2015:1–13. https://doi.org/10.1155/2015/519513.
-
Melis A. Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009;177(4):272–80. https://doi.org/10.1016/j.plantsci.2009.06.005.
-
Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Maréchal-Drouard L, Marshall WF, Qu L-H, Nelson DR, Sanderfoot AA, Spalding MH, Kapitonov VV, Ren Q, Ferris P, Lindquist E, Shapiro H, Lucas SM, Grimwood J, Schmutz J, Cardol P, Cerutti H, Chanfreau G, Chen CL, Cognat V, Croft MT, Dent R, Dutcher S, Fernández E, Fukuzawa H, González-Ballester D, González-Halphen D, Hallmann A, Hanikenne M, Hippler M, Inwood W, Jabbari K, Kalanon M, Kuras R, Lefebvre PA, Lemaire SD, Lobanov AV, Lohr M, Manuell A, Meier I, Mets L, Mittag M, Mittelmeier T, Moroney JV, Moseley J, Napoli C, Nedelcu AM, Niyogi K, Novoselov SV, Paulsen IT, Pazour G, Purton S, Ral JP, Riaño-Pachón DM, Riekhof W, Rymarquis L, Schroda M, Stern D, Umen J, Willows R, Wilson N, Zimmer SL, Allmer J, Balk J, Bisova K, Chen CJ, Elias M, Gendler K, Hauser C, Lamb MR, Ledford H, Long JC, Minagawa J, Page MD, Pan J, Pootakham W, Roje S, Rose A, Stahlberg E, Terauchi AM, Yang P, Ball S, Bowler C, Dieckmann CL, Gladyshev VN, Green P, Jorgensen R, Mayfield S, Mueller-Roeber B, Rajamani S, Sayre RT, Brokstein P, Dubchak I, Goodstein D, Hornick L, Huang YW, Jhaveri J, Luo Y, Martínez D, Ngau WC, Otillar B, Poliakov A, Porter A, Szajkowski L, Werner G, Zhou K, Grigoriev IV, Rokhsar DS, Grossman AR. The chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318(5848):245–50. https://doi.org/10.1126/science.1143609.
-
Mertins A, Wawer T. How to use biogas?: A systematic review of biogas utilization pathways and business models. Bioresour Bioprocess. 2022. https://doi.org/10.1186/s40643-022-00545-z.
-
Milledge JJ, Heaven S. Methods of energy extraction from microalgal biomass: a review. Rev Environ Sci Bio/Technol. 2014;13:301–20. https://doi.org/10.1007/s11157-014-9339-1.
-
Muhammad U. Biofuels as the starring substitute to fossil fuels. Pet Sci Engin. 2018;2:44. https://doi.org/10.11648/j.pse.20180201.17.
-
Naduthodi MIS, Mohanraju P, Südfeld C, D’Adamo S, Barbosa MJ, Van Der Oost J. CRISPR–cas ribonucleoprotein mediated homology-directed repair for efficient targeted genome editing in microalgae Nannochloropsis oceanica IMET1. Biotechnol Biofuels. 2019. https://doi.org/10.1186/s13068-019-1401-3.
-
Neeti K, Gaurav K, Singh R. The potential of algae biofuel as a renewable and sustainable bioresource. Engin Proceed. 2023;37:22. https://doi.org/10.3390/ECP2023-14716.
-
Nigam PS, Singh A. Production of liquid biofuels from renewable resources. Prog Energ Combust Sci. 2010;37(1):52–68. https://doi.org/10.1016/j.pecs.2010.01.003.
-
Nomura T, Inoue K, Uehara-Yamaguchi Y, Yamada K, Iwata O, Suzuki K, Mochida K. Highly efficient transgene-free targeted mutagenesis and single-stranded oligodeoxynucleotide-mediated precise knock-in in the industrial microalga Euglena gracilis using Cas9 ribonucleoproteins. Plant Biotechnol J. 2019;17(11):2032–4. https://doi.org/10.1111/pbi.13174.
-
O’Loughlin JL, Doherty DC, Herward B, McGleenan C, Mahmud M, Bhagabati P, Boland AN, Freeland B, Rochfort KD, Kelleher SM, Fahy S, Gaughran J. The potential of bio-based polylactic acid (PLA) as an alternative in reusable food containers: a review. Sustainability. 2023;15:15312–15312. https://doi.org/10.3390/su152115312.
-
O’Neill BM, Mikkelson KL, Gutierrez NM, Cunningham JL, Wolff KL, Szyjka SJ, Yohn CB, Redding KE, Mendez MJ. An exogenous chloroplast genome for complex sequence manipulation in algae. Nucleic Acids Res. 2011;40(6):2782–92. https://doi.org/10.1093/nar/gkr1008.
-
Osman M, Xiaohou S, Zhao D, Basheer A, Jin H, Zhang Y. Methane production from alginate-extracted and non-extracted waste of laminaria japonica: anaerobic mono- and synergetic co-digestion effects on yield. Sustainability. 2019;11:1269. https://doi.org/10.3390/su11051269.
-
Panahi B, Frahadian M, Dums JT, Hejazi MA. Integration of cross species RNA-SEQ meta-analysis and machine-learning models identifies the most important salt stress-responsive pathways in microalga dunaliella. Front Genet. 2019. https://doi.org/10.3389/fgene.2019.00752.
-
Pandey S, Narayanan I, Selvaraj R, Varadavenkatesan T, Vinayagam R. Biodiesel production from microalgae: a comprehensive review on influential factors, transesterification processes, and challenges. Fuel. 2024;367:131547–131547. https://doi.org/10.1016/j.fuel.2024.131547.
-
Paniagua-Michel J, Farfan BC, Bückle-Ramirez F. Culture of marine microalgae with natural biodigested resources. Aquaculture. 1987;64(3):249–56. https://doi.org/10.1016/0044-8486(87)90330-9.
-
Park H, He H, Yan X, Liu X, Scrutton NS, Chen G-Q. PHA is not just a bioplastic! Biotechnol Adv. 2024;71:108320–108320. https://doi.org/10.1016/j.biotechadv.2024.108320.
-
Park J, Kim J-K, Park C. A review of biofuels production technologies from microalgae. Trans Korean Hydrogen New Energy Soc. 2016;27:386–403. https://doi.org/10.7316/khnes.2016.27.4.386.
-
Peltomaa E, Johnson M, Taipale S. Marine cryptophytes are great sources of EPA and DHA. Mar Drugs. 2017;16:3. https://doi.org/10.3390/md16010003.
-
Perdana BA, Chaidir Z, Kusnanda AJ, Dharma A, Zakaria IJ, Syafrizayanti, Bayu A, Putra MY. Omega-3 fatty acids of microalgae as a food supplement: a review of exogenous factors for production enhancement. Algal Res. 2021;60:102542. https://doi.org/10.1016/j.algal.2021.102542.
-
Pimentel F, Alves R, Rodrigues F, Oliveira PP, Beatriz M. Macroalgae-derived ingredients for cosmetic industry—an update. Cosmetics. 2017;5:2. https://doi.org/10.3390/cosmetics5010002.
-
Pittman JK, Dean AP, Osundeko O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol. 2011;102:17–25. https://doi.org/10.1016/j.biortech.2010.06.035.
-
Powell EE, Hill GA. Economic assessment of an integrated bioethanol–biodiesel–microbial fuel cell facility utilizing yeast and photosynthetic algae. Process Saf Environ Prot. 2009;87(9):1340–8. https://doi.org/10.1016/j.cherd.2009.06.018.
-
Prasad R, Gupta SK, Shabnam N, Oliveira CYB, Nema AK, Ansari FA, Bux F. Role of microalgae in global CO2 sequestration: physiological mechanism, recent development, challenges, and future prospective. Sustainability. 2021;13:13061. https://doi.org/10.3390/su132313061.
-
Ramasamy S, Arun S, Pakshirajan K, Singh L, Yousuf A, Mahapatra DM. Chapter 13: an overview of algal photobioreactors for resource recovery from waste. In: bioreactors. Amsterdam: Elsevier; 2020. p. 215–48.
-
Razeghifard R. Algal biofuels. Photosynth Res. 2013;117(1–3):207–19. https://doi.org/10.1007/s11120-013-9828-z.
-
Rehman ZU, Anal AK. Enhanced lipid and starch productivity of microalga (Chlorococcum sp. TISTR 8583) with nitrogen limitation following effective pretreatments for biofuel production. Biotechnol Rep (Amst). 2019;21: e00298. https://doi.org/10.1016/j.btre.2018.e00298.
-
Ruiz-Marin A, Canedo-Lopez Y, Chavez-Fuentes P. Biohydrogen production by Chlorella vulgaris and Scenedesmus obliquus immobilized cultivated in artificial wastewater under different light quality. AMB Express. 2020;10(1):191. https://doi.org/10.1186/s13568-020-01129-w.
-
Ruiz J, Wijffels RH, Dominguez M, Barbosa MJ. Heterotrophic vs autotrophic production of microalgae: bringing some light into the everlasting cost controversy. Algal Res. 2022;64: 102698. https://doi.org/10.1016/j.algal.2022.102698.
-
Ryckebosch E, Bruneel C, Muylaert K, Foubert I. Microalgae as an alternative source of omega-3 long chain polyunsaturated fatty acids. Lipid Technol. 2012;24:128–30. https://doi.org/10.1002/lite.201200197.
-
Saad MG, Dosoky NS, Zoromba MS, Shafik HM. Algal biofuels: current status and key challenges. Energies. 2019;12(10):1920. https://doi.org/10.3390/en12101920.
-
Seon G, Kim HS, Cho JM, Kim M, Park W-K, Chang YK. Effect of post-treatment process of microalgal hydrolysate on bioethanol production. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-73816-4.
-
Serry H, Hassanien AE, Zaghlou S, Hefny HA. Predicting algae growth in the Nile River using meta-learning techniques. Berlin: Springer; 2017.
-
Shahid A, Malik S, Khan AZ, Liu CG, Mehmood M. Multiproduct algal biorefineries: challenges and opportunities. Berlin: Springer; 2020. p. 513–37.
-
Sheng J, Vannela R, Rittmann BE. Evaluation of cell-disruption effects of pulsed-electric-field treatment of Synechocystis PCC 6803. Environ Sci Technol. 2011;45(8):3795–802. https://doi.org/10.1021/es103339x.
-
Show K-Y, Yan Y, Ling M, Ye G, Li T, Lee D-J. Hydrogen production from algal biomass – advances, challenges and prospects. Bioresour Technol. 2018;257:290–300. https://doi.org/10.1016/j.biortech.2018.02.105.
-
Sialve B, Bernet N, Bernard O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol Adv. 2009;27:409–16. https://doi.org/10.1016/j.biotechadv.2009.03.001.
-
Singh A, Nigam PS, Murphy JD. Mechanism and challenges in commercialisation of algal biofuels. Bioresour Technol. 2010;102(1):26–34. https://doi.org/10.1016/j.biortech.2010.06.057.
-
Singh A, Nigam PS, Murphy JD. Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol. 2011;102:10–6. https://doi.org/10.1016/j.biortech.2010.06.032.
-
Singh A, Olsen SI. A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Appl Energy. 2011;88(10):3548–55. https://doi.org/10.1016/j.apenergy.2010.12.012.
-
Smith VH, Sturm BSM, deNoyelles FJ, Billings SA. The ecology of algal biodiesel production. Trends Ecol Evol. 2009;25(5):301–9. https://doi.org/10.1016/j.tree.2009.11.007.
-
Souza CMM, Bastos TS, Santos MCd. Microalgae use in animal nutrition. Res Soc Dev. 2021;10: e53101622986. https://doi.org/10.33448/rsd-v10i16.22986.
-
Subhadra B, Grinson-George N. Algal biorefinery-based industry: an approach to address fuel and food insecurity for a carbon-smart world. J Sci Food Agric. 2010;91(1):2–13. https://doi.org/10.1002/jsfa.4207.
-
Sulfahri AM, Sumitro SB, Saptasari M. Bioethanol production from algae spirogyra hyalina using zymomonas mobilis. Biofuels. 2016;7(6):621–6. https://doi.org/10.1080/17597269.2016.1168028.
-
Teng SY, Loy ACM, Leong WD, How BS, Chin BLF, Máša V. Catalytic thermal degradation of Chlorella vulgaris: evolving deep neural networks for optimization. Bioresour Technol. 2019;292: 121971. https://doi.org/10.1016/j.biortech.2019.121971.
-
Tham PE, Ng YJ, Vadivelu N, Lim HR, Khoo KS, Chew KW, Show PL. Sustainable smart photobioreactor for continuous cultivation of microalgae embedded with internet of things. Biores Technol. 2021;346: 126558. https://doi.org/10.1016/j.biortech.2021.126558.
-
Torzillo G, Scoma A, Faraloni C, Giannelli L. Advances in the biotechnology of hydrogen production with the microalga Chlamydomonas reinhardtii. Crit Rev Biotechnol. 2014;35:485–96. https://doi.org/10.3109/07388551.2014.900734.
-
Trivedi J, Aila M, Bangwal DP, Kaul S, Garg MO. Algae based biorefinery—how to make sense? Renew Sustain Energy Rev. 2015;47:295–307. https://doi.org/10.1016/j.rser.2015.03.052.
-
Uggetti E, Sialve B, Trably E, Steyer J-P. Integrating microalgae production with anaerobic digestion: a biorefinery approach. Biofuels Bioprod Biorefin. 2014;8:516–29. https://doi.org/10.1002/bbb.1469.
-
Ullah K, Ahmad M, Sofia N, Sharma VK, Lu P, Harvey A, Zafar M, Sultana S, Anyanwu CN. Algal biomass as a global source of transport fuels: overview and development perspectives. Progr Nat Sci Mater Int. 2014;24(4):329–39. https://doi.org/10.1016/j.pnsc.2014.06.008.
-
Ullmann J, Grimm D. Algae and their potential for a future bioeconomy, landless food production, and the socio-economic impact of an algae industry. Org Agric. 2021;11(2):261–7. https://doi.org/10.1007/s13165-020-00337-9.
-
Ungureanu N, Vladut V, Biris S. Capitalization of wastewatergrown algae in bioethanol production. Engin Rur Dev. 2020. https://doi.org/10.22616/ERDev2020.19.TF507.
-
Vasić K, Knez Ž, Leitgeb M. Bioethanol production by enzymatic hydrolysis from different lignocellulosic sources. Molecules. 2021. https://doi.org/10.3390/molecules26030753.
-
Velasquez-Orta SB, Lee JGM, Harvey AP. Evaluation of FAME production from wet marine and freshwater microalgae by in situ transesterification. Biochem Eng J. 2013;76:83–9. https://doi.org/10.1016/j.bej.2013.04.003.
-
Velazquez-Lucio J, Rodríguez-Jasso RM, Colla LM, Sáenz-Galindo A, Cervantes-Cisneros DE, Aguilar CN, Fernandes BD, Ruiz HA. Microalgal biomass pretreatment for bioethanol production: a review. Biofuel Res J. 2018;5:780–91. https://doi.org/10.18331/brj2018.5.1.5.
-
Vilalta R, Drissi Y. A perspective view and survey of meta-learning. Artif Intell Rev. 2002;18(2):77–95. https://doi.org/10.1023/a:1019956318069.
-
Voloshin RA, Rodionova MV, Zharmukhamedov SK, Veziroglu TN, Allakhverdiev SI. Biofuel production from plant and algal biomass. Int J Hydrogen Energy. 2016;41(39):17257–73. https://doi.org/10.1016/j.ijhydene.2016.07.084.
-
Wang J, Yin Y. Fermentative hydrogen production using pretreated microalgal biomass as feedstock. Microb Cell Fact. 2018. https://doi.org/10.1186/s12934-018-0871-5.
-
Winwood RJ, Jacobsen C, Nielsen NS, Horn AF, Sorensen AM. Algal oil as a source of omega-3 fatty acids. New Delhi: Woodhead Publishing; 2013. p. 389–404.
-
Yadav K, Vasistha S, Nawkarkar P, Kumar S, Rai MP. Algal biorefinery culminating multiple value-added products: recent advances, emerging trends, opportunities, and challenges. 3 Biotech. 2022. https://doi.org/10.1007/s13205-022-03288-y.
-
Yao Y, Xu B. Skin health promoting effects of natural polysaccharides and their potential application in the cosmetic industry. Polysaccharides. 2022;3:818–30. https://doi.org/10.3390/polysaccharides3040048.
-
Yoshimitsu Y, Abe J, Harayama S. Cas9-guide RNA ribonucleoprotein-induced genome editing in the industrial green alga Coccomyxa sp. strain KJ. Biotechnol Biofuels. 2018. https://doi.org/10.1186/s13068-018-1327-1.
-
Zabochnicka M, Krzywonos M, Romanowska-Duda Z, Szufa S, Darkalt A, Mubashar M. Algal biomass utilization toward circular economy. Life. 2022. https://doi.org/10.3390/life12101480.
-
Zaimes GG, Khanna V. Environmental sustainability of emerging algal biofuels: a comparative life cycle evaluation of algal biodiesel and renewable diesel. Environ Prog Sustainable Energy. 2013;32:926–36. https://doi.org/10.1002/ep.11810.
-
Zamora-Castro J, Paniagua-Michel J, Lezama-Cervantes C. A novel approach for bioremediation of a coastal marine wastewater effluent based on artificial microbial mats. Mar Biotechnol. 2007;10(2):181–9. https://doi.org/10.1007/s10126-007-9050-0.
-
Zhu LD, Li ZH, Hiltunen E. Strategies for lipid production improvement in microalgae as a biodiesel feedstock. Biomed Res Int. 2016;2016:1–8. https://doi.org/10.1155/2016/8792548.
-
Zuorro A, Maffei G, Lavecchia R. Kinetic modeling of azo dye adsorption on non-living cells of Nannochloropsis oceanica. J Environ Chem Eng. 2017;5(4):4121–7. https://doi.org/10.1016/j.jece.2017.07.078.
Funding
This research was funded by Ministry of Education, China (S202514389095; 202514389038) and Chengdu Normal University (2024XJCXXSY3).
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Geng, Y., Shaukat, A., Azhar, W. et al. Microalgal biorefineries: a systematic review of technological trade-offs and innovation pathways. Biotechnol. Biofuels Bioprod. 18, 93 (2025). https://doi.org/10.1186/s13068-025-02694-7
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1186/s13068-025-02694-7