Introduction
Antimicrobial resistance (AMR) in mastitis-associated pathogens (e.g., Staphylococcus aureus, Escherichia coli) is a major public health concern due to its zoonotic transmission via raw milk consumption1,2,3,4,5. Resistant strains complicate mastitis treatment, often leading to prolonged antibiotic use in dairy herds, which further drives AMR emergence6. A high somatic cell count (SCC) is indicative of mastitis, an inflammatory condition often triggered by bacterial infection, leading to subclinical mastitis, a condition frequently managed with empiric antibiotics, thus underscoring the need for rapid SCC monitoring to reduce unnecessary antibiotic use7,8. Raw milk can harbor a wide range of harmful microorganisms, including Salmonella, Escherichia coli O157, Listeria monocytogenes, Campylobacter jejuni, Staphylococcus aureus, and various species of Streptococcus and Klebsiella, all of which are known to cause foodborne illnesses9,10,11,12,13,14,15,16,17.
The presence of resistant pathogens in milk increases the risk of bacterial outbreaks, posing severe public health consequences, particularly for vulnerable populations such as children, the elderly, and immunocompromised individuals18,19,20,21. Emerging surveillance reports underscore the role of dairy products in foodborne disease outbreaks linked to AMR pathogens, further necessitating field-level screening and mitigation strategies22,23,. Recent studies have explored novel AMR monitoring tools, such as CRISPR-Cas-based detection24 and nanopore sequencing for real-time resistance gene profiling25. However, these methods remain confined to laboratory settings, leaving a critical gap in field-deployable AMR screening. The European Food Safety Authority26,27 reported that 5.1% of foodborne outbreaks were linked to contaminated cheese, while the U.S. documented 208 milk-related outbreaks (1987–2018) due to unpasteurized milk28,29.
The SCC in milk is a globally recognized indicator of microbial quality and mastitis30. Somatic cells (SCs) infiltrate the milk as a defense mechanism against pathogenic invasion in the mammary glands. Elevated SCC not only predicts microbial contamination but also negatively impacts milk composition and quality, which correlates with altered milk composition (e.g., lactose, casein) and sensory defects31,32,33,34,35. The lactose content in milk from cows with mastitis gets reduced from 4.86 to 4.69% with an increase in SCC from 104 to 4.5 × 105 cells/mL36,37. Additionally, it contributes to off-flavoring and shortening of shelf life due to increased enzymatic activity from both somatic cells and bacteria. Degradation of milk fat and protein impart rancid and bitter flavors thereby compromising the sensory quality of dairy products38,39,40.
Although SCC measurement methods such as microscopy, flow cytometry, and electric impulse analysis are effective, they are impractical for field use, particularly in developing countries where milk production often occurs in unregulated, decentralized sectors lacking access to centralized diagnostics41,42,43,44,45,46. Recent advances in portable SCC detection include smartphone-based optical assays42, microfluidic sensors47, and AI-driven image analysis41. Despite innovations like biosensor-based SCC quantification4,48 and miniaturized impedance cytometers49, no existing device combines somatic cell detection with AMR assessment for on-farm use. Though these technologies are promising, they face limitations in cost, scalability, or integration with AMR screening for decentralized dairy sectors in developing regions, underscoring the need for a unified, field-adaptable solution.
To bridge this gap, we introduce “QuantM”—a field-deployable diagnostic device combining Iron oxide nanoparticle-based somatic cell aggregation with a low-cost optical reader and image processing algorithm. This device enables rapid and quantitative SCC analysis while laying the groundwork for future AMR detection modules, empowering dairy stakeholders to mitigate mastitis and AMR risks at the point-of-milk collection.
Results
Synthesis and characterization of positively charged IONP
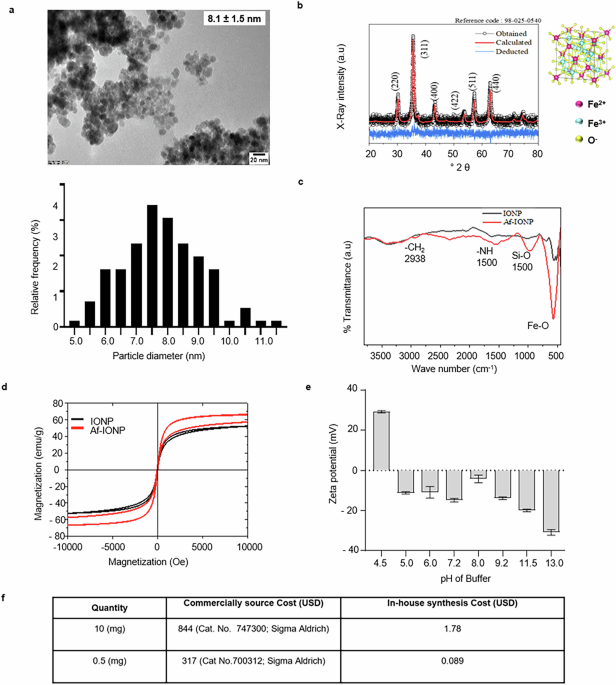
The Iron oxide nanoparticles (IONP) were synthesized using the reverse precipitation method and amine functionalized using APTES as mentioned in Methods. Furthermore, the amine-functionalized iron oxide nanoparticles (Af-IONP) were characterized for morphological, physical, and chemical properties. TEM image revealed the synthesis of spherical particles with a particle size of ~8.1 ± 1.5 nm (Fig. 1a). The crystalline nature of the particles was determined through X-ray diffraction analysis. Diffraction peaks at 2θ values of 30.8°, 36.4°, 43.5°, 54.6°, 56.8°, and 62.3° were indexed to be (111), (220), (311), (222), (400), (422), and (511) planes, respectively. These particles belong to the space group Fd-3m with lattice parameters a = b = c = 8.3910 Å. After Rietveld refinement, the linear fit between the observed and calculated diffraction patterns showed no additional peaks, confirming the formation of magnetite (Fe3O4) and matching the standard X-ray diffraction (XRD) pattern for Fe3O4 (JCPDS card no. 01-071-6337; Fig. 1b). Using Eq. 1, the crystallite size of the particle was determined to be 8.5 nm.
a Transmission electron microscopy (TEM) image showing spherical nanoparticles with a particle size (8.1 ± 1.5 nm) along with the distribution of particle size. b The X-ray diffractogram showing the phase formation as magnetite (Fe3O4) with a cubic crystal structure. c FT-IR spectrum of amine-functionalization of IONP with characteristic peaks of N-H bond at 1560 cm−1 wavenumber. d Magnetic hysteresis loop of the IONP before and after amine functionalization; saturation magnetization, coercivity, and remanence parameters of the hysteresis loop confirm the superparamagnetic property of IONP. e Effect of varying pH on the charge of Af-IONP. f Table highlighting the cost comparison of the in-house synthesized Af-IONP as compared to the commercial source.
Further, the Fourier transform-infrared (FT-IR) spectrum of Af-IONP showed characteristic peaks at 562, 997, 1560, and 2938 cm−1 corresponding to Fe-O, Si-O, N-H, and CH2, respectively (Fig. 1c). The dried IONP before and after amine functionalization showed a strong magnetization characteristic for its superparamagnetic nature (Fig. 1d) with less coercivity and remanence at the field of 10 kOe in the vibrating sample magnetometer (Supplementary Fig. 1). The Af-IONP were suspended in different buffers to stabilize the positive charge (Fig. 1e). The surface charge of the particles suspended in acetate buffer (10 mM; pH 4.5) retained positive charge while particles suspended in other buffers (from 5 to 13) gained negative charge (Fig. 1e).
IONP-based assay for the detection of somatic cells
Incubation of positively charged Af-IONP with mammary epithelial (HC11) cells resulted in nanoparticle deposition on the cell surface, as confirmed by TEM (Fig. 2a). A significant (p < 0.005) increase in the hydrodynamic size of the cell–Af-IONP complex (3684.0 ± 266.9 nm) was observed compared to individual HC11 cells (1627.6 ± 103.0 nm) and Af-IONP (218.0 ± 30 nm) (Fig. 2b). Additionally, the zeta potential of the Af-IONP showed a significant (p < 0.005) reduction from 24 ± 2 mV to 11.2 ± 2.7 mV upon interaction with the cells (Fig. 2c). Optimal conditions for effective cell sequestration were determined by varying the nanoparticle concentration and incubation time.
a TEM images showing the interaction of iron oxide nanoparticles with cells. b Zeta potential and c hydrodynamic diameter of iron oxide nanoparticles, cells, and cell-iron oxide nanoparticle complexes. d In 96-well plate, number of cells were increased row-wise while the amine-functionalized iron oxide nanoparticles were varied column-wise. After 10 minutes of incubation, protein content was measured in the supernatant (i). The graph shows the protein content in the supernatant of different wells of the 96 well plate (ii). e The sequestration was performed at various time intervals at constant cell (0.1 million) number to optimize the incubation time (i) The graph shows the protein content in supernatant and pellet as a function of time (ii). f Aggregate formation in cell-iron oxide nanoparticle mixtures treated with different concentrations of surfactants like Tween-20, Triton, SDS, CTAB, Saponin at various concentrations. Significant aggregation was observed at 0.25% SDS concentration, as noted with densitometric analysis (ii). g Aggregation response with varying numbers of epithelial cells in PBS (200 µL; 10 mM), proportionately increasing aggregation with cells. h Specificity of aggregation tested with various milk components (DNA, proteins, bacteria, cells). Aggregation was observed with epithelial cells and a positive control sample (milk from an infected animal). i Aggregation formation with different fractions of milk by centrifugation and j filtration. Aggregation was observed with the pellet fraction of centrifugation and the retentate fraction of filtration.
A progressive decrease in protein content in the supernatant was observed with increasing nanoparticle concentration at a fixed number of HC11 cells (Fig. 2d). This trend was apparent even after just one minute of incubation (Fig. 2e). Optimization studies indicated that the optimal results were achieved using 500 µg of Af-IONP with an incubation time of one minute.
Surfactant-induced formation of cell–Af-IONP aggregates
We investigated the effect of various surfactants on the aggregation of mammary epithelial cells (HC11) sequestered with Af-IONP. Among the surfactants tested, 0.25% SDS uniquely facilitated the formation of visibly detectable aggregates (Fig. 2f). Notably, aggregate formation exhibited a positive correlation with cell number, with visible aggregation occurring at cell counts as low as 0.05 million cells/mL (Fig. 2g).
Cell-specific aggregation in milk components
Having established SDS-mediated aggregation, we next verified the assay’s specificity toward somatic cells amidst milk’s complex composition. Testing four biologically relevant components—proteins (casein/whey), DNA, bacteria (E. coli), and HC11 (representative of somatic cells)—revealed striking selectivity (Fig. 2h). Only somatic cells (0.05–0.1 million cells/mL) induced rapid aggregation, with TEM confirming nanoparticles bound exclusively to cell membranes (Fig. 2a). This selectivity persisted in raw milk spiked with 10⁶ CFU/mL bacteria, protein and DNA, demonstrating robustness against common interferents. The intensity of aggregates formed with HC11 cells was established through densitometry, supporting SCs as a factor for the formation of aggregates (Fig. 2h).
To further validate the specificity of cell-nanoparticle aggregation, we fractionated aggregation-positive milk samples through centrifugation and filtration. The aggregation analyses on these fractions revealed that nanoparticle aggregates were exclusively present in the centrifuged pellet fraction (Fig. 2i) and the filtration retentate (Fig. 2j). No aggregates were detected in either the centrifugation supernatant or the filtration flow-through. These results demonstrate that intact somatic cells are required for nanoparticle-mediated aggregation and that soluble milk components do not contribute to aggregate formation.
Design and development of a QuantM device for SCC
Magnetic-assisted cell concentrator (MACC) is a key component of the QuantM device, consisting of a cylindrical base with a bar magnet and a sample holder designed with circular extrusions to secure sample tubes. Its ergonomic design enabled the simultaneous processing of two samples. The semi-rotatory sample holder allowed the tubes to be positioned either towards or away from the magnets during the procedure, ensuring efficient operation (Fig. 3a). The preview of the 3D device models in the slicer interface is presented in Supplementary Fig. 2.
a Design and fabrication of the MACC, consisting of a cylindrical base with bar magnets and a sample holder with circular extrusions for sample tubes. The semi-rotatory movement of the sample holder allows sample positioning towards or away from the magnets. b Setup of the AVM, which includes a nitrocellulose membrane (NCM) backed by an absorbent pad to create capillary force. c SEM images of NCMs with different pore sizes (0.2, 0.3, 0.45, 0.8 µm). The 0.8 µm membrane was selected for optimal aggregate retention. d Visualization of cell aggregates on the 0.8 µm NCM of the AVM with increasing epithelial cell concentrations in PBS (10 mM) and (e) milk. f Retention of aggregates on the surface of NCM (0.8 µm) as shown by SEM. g Assembly of the 3D-printed image acquisition module for AVM imaging is designed to capture clear images even in low-light conditions. h Image Processing Algorithm Flowchart showing step-by-step process of the image analysis algorithm used to quantify epithelial cell counts. i Captured AVM images are converted to grayscale, j threshold converted, and analyzed for percentage black pixels, scaled by a factor of 100 for enhanced precision. k Linear regression plot of black pixel percentage against known somatic cell counts (0 to 200 × 10³ cells/mL), with a derived slope of 0.0017 and an intercept of 28 incorporated into the algorithm for cell count estimation. l (i)-Block diagram of AVM reader integrated with Raspberry Pi 4, camera module, and a 7-inch touchscreen display and other components. Assembled AVM reader. (ii) Portable AVM Reader for Field Use.
The AVM was developed using a nitrocellulose membrane (NCM) backed by an absorbent pad capable of soaking fluids while retaining the aggregates on the NCM’s surface (Fig. 3b). The NCM was selected for its fast flow rate to facilitate the separation of free Af-IONP. Testing revealed that a 0.8 µm NCM exhibited faster fluid absorption (Fig. 3c). The membrane achieved full sample absorption within 45 ± 12 seconds (n = 30), demonstrating rapid and consistent performance (Supplementary Fig. 7). Using this NCM, the AVM was evaluated for its ability to retain cell–Af-IONP aggregates formed during aggregation tests performed in buffer and milk at varying cell concentrations. A consistent pattern of aggregate retention was observed across triplicates, which was further confirmed through SEM images, showing large patches of nanoparticle-cell aggregates on the membrane. Supplementary Fig. 3a, b presents the photographs of the MACC, AVM.
The QuantM workflow integrates the MACC and AVM components, with the application of the sample onto the AVM’s NCM. This setup effectively retains cell aggregates, allowing visualization in field conditions. When tested with varying epithelial cell concentrations in PBS buffer (Fig. 3d) and milk (Fig. 3e), the assay reliably detected aggregates from a concentration as low as 0.05 million cells/mL. SEM images further validated the AVM’s ability to capture and retain aggregates on the 0.8 µm NCM, underscoring its efficiency and utility in cell detection applications (Fig. 3f).
Development of an image processing algorithm and AVM reader for quantitative somatic cell counting
To achieve quantitative somatic cell counting from the extent of aggregation, an AVM reader with a dedicated camera module integrated with a Raspberry Pi 4 motherboard and a 7-inch touchscreen display was made using 3D printing to capture images of the aggregates on the AVM. The camera module ensured repeatable and uniform image resolution by optimizing focal distance and angle. It incorporated a CMOS camera and LED illumination to facilitate consistent, high-quality image capture (Fig. 3g). An image-processing algorithm (Fig. 3h) was developed and trained using datasets derived from AVMs of aggregation assays conducted with milk somatic cells (SCs) spiked into cell-free milk at varying cell concentrations. The algorithm acquires images using defined coordinates, aligning the camera frame with the region of interest (ROI) (Fig. 3i). The selected frame is converted to grayscale, and a threshold is applied to quantify the percentage of black pixels, which is then scaled 100-fold to enhance measurement precision (Fig. 3j). A standard curve was established and integrated the slope value (a = 0.0017 and intercept = 28) from the equation generated in the linear fit (y = a.x + c) with R2 = 0.98 (Fig. 3k). After that, AVM reader device has been developed that operates on Raspbian OS and includes essential libraries (Flask, Open CV, NumPy, etc.). The accompanying web application running on local server (Supplementary Fig. 4a), developed using Python-Flask and Bootstrap, will allow the users to upload AVM images (Supplementary Fig. 4b), crop to the ROI, and process the image for quantification through pre-installed reader assembled components (Fig. 3l). The results will be displayed in the UI with the somatic cell count and the uploaded and processed image (Supplementary Fig. 4c).
Upon booting, the AVM reader will launch a local web server to display a user-friendly web application through Chromium (a browser of Raspbian Operating system) thereby enabling the users to on-site capture and analyze the AVM images (Supplementary Fig. 5). This seamless integration ensured the reader’s usability and accuracy under field conditions, enabling reliable and efficient somatic cell counting (Supplementary Fig. 3c, d).
Comparative efficacy of QuantM device for somatic cell detection in spiked and clinical samples
The performance of QuantM device was compared for somatic cell detection with respect to gold-standard flow cytometry. Milk somatic cells were spiked into cell-free milk, and the results were analyzed using both methods following aggregation assay procedure (Fig. 4a). Histograms of the flow cytometry results and QuantM readouts demonstrated a strong correlation, as shown in Fig. 4b. Furthermore, correlation of QuantM and flow cytometry data were showed >90% correlation at each concentration of cells (Fig. 4c). Linear regression analysis between the two methods yielded a high correlation coefficient (R2 = 0.99), with a slope value of 0.92. The slope value has been used to determine the limit of detection of the AVM reader, calculated to be 0.017 million cells/mL (Fig. 4d). Bland-Altman analysis revealed minimal systematic bias, with a mean bias of 0.00092 and a standard deviation of 0.002857. The 95% limits of agreement ranged from −0.000920, encompassing 95% of the differences between the two methods, indicating high agreement (Fig. 4e). An increase in somatic cell count led to a visible rise in IONP aggregate formation on the AVM membrane, which could even be observed with the naked eye. This visual confirmation further supports the quantitative findings.
a Workflow showing the QuantM for the assessment of milk quality. b Representative images displaying histograms of Thiazole Orange-positive events (blue) obtained via flow cytometry, alongside AVM images. The readout for both methods is shown above each respective image. c Table showing the percent correlation of somatic cell counts at different cell numbers between flow cytometry and QuantM. d Linear regression plot demonstrating a strong correlation (R2 = 0.99) between the two methods. e Bland–Altman plot highlighting the excellent agreement between QuantM and flow cytometry in milk samples spiked with increasing somatic cell concentrations. f Spearman correlation showing a high degree of agreement (r = 0.94, P < 0.0001) between aggregation data from QuantM and flow cytometry when tested on field-collected samples.
To validate the device in real-world conditions, 60 milk samples were collected and analyzed using both the AVM reader and flow cytometry (Supplementary Fig. 6). The Spearman correlation coefficient between the somatic cell counts obtained from the two methods was r = 0.94 (P < 0.0001), confirming a strong relationship (Fig. 4f). The AVM reader achieved 94.74% sensitivity, 90.0% specificity, and an overall accuracy of 94.74%. The 95% confidence intervals for sensitivity, specificity, and accuracy further reinforce the reliability of the AVM reader for field-based somatic cell detection. This validation demonstrated the effectiveness of the QuantM device in quantifying somatic cells with precision, making it a robust alternative for rapid, field-deployable somatic cell detection in milk samples.
Discussion
The present study addresses the critical need for a point-of-care system to detect somatic cells in milk, an essential indicator of milk quality and safety, particularly in developing countries where food safety regulations and testing infrastructure are often inadequate50,51,52. Early and cost-effective monitoring of SCC not only ensures milk quality but also indirectly contributes to AMR mitigation by reducing overuse of antibiotics in response to undiagnosed mastitis6,15. This work represents a significant advancement over existing methods for synthesizing functionalized IONP. As compared to conventional synthesis methods, which are often costly and require specialized conditions, this approach is more accessible and scalable. The functionalization of IONP with APTES provided a stable positive charge under acidic conditions (Fig. 1e), enabling versatile applications in aggregation assays, diagnostics, and other biomedical fields53. The superparamagnetic nature of these particles further enhances their usability in magnetic separation techniques, reducing reliance on complex equipment and allowing for faster, more efficient workflows54,55,56. The reverse precipitation method combined with amine functionalization offers a straightforward, cost-effective process that can produce 10 grams of nanoparticles at a cost of <$2.0 (Fig. 1f). This combination of affordability and functionality makes these nanoparticles particularly advantageous for applications in resource-limited settings.
The use of Af-IONP offers several advantages for somatic cell detection in milk, providing a significant improvement over existing methods reported in the literature for bacterial and fungal capture57. The positively charged amine groups on the nanoparticle surface facilitate strong electrostatic interactions with the negatively charged membranes of somatic cells, as confirmed by TEM image (Fig. 2a) and supported by dynamic light scattering measurements of hydrodynamic size (Fig. 2b) and zeta potential (Fig. 2c). While this principle of electrostatic interaction has previously been utilized for bacterial sequestration at ultralow concentrations58,59,60,61,62, its adaptation for somatic cell capture represents a novel repurposing with substantial diagnostic implications for the dairy industry. The synthesized Af-IONP exhibited a robust positive charge, which enhanced their interaction with the negatively charged cell membranes of mammalian cells at physiological pH. This interaction formed the cornerstone of the aggregation-based assay, enabling rapid and efficient cell sequestration. The optimized parameters (Fig. 2d, e) demonstrated their speed and practicality, successfully sequestering cells in milk samples.
Importantly, the innovative aspect of the QuantM methodology lies in its integration of nanoparticle-mediated sequestration with surfactant-assisted aggregation. This can be explained by Derjaguin–Landau–Verwey–Overbeek (DLVO) theory, which describes the interplay between electrostatic repulsion and van der Waals attraction for colloidal stability of particles63,64,65. The Af-IONPs, with a +24 mV ζ-potential, are highly stable due to strong electrostatic repulsion. This stability prevents spontaneous aggregation in the presence of cells. We found that 0.25% SDS, an anionic surfactant, effectively reduces surface charge by neutralizing the positive ζ-potential through its sulfate groups. This reduction in electrostatic repulsion allows van der Waals forces to dominate, promoting particle-cell aggregation. This interaction creates a crosslink network, forming stable, shear-resistant aggregates. SDS, uniquely, balances charge neutralization with effective hydrophobic integration63. The resulting dense aggregates enhance detection sensitivity down to 0.05 million cells/mL (Fig. 2g). By carefully modulating the DLVO interaction landscape, QuantM achieves a controlled and robust aggregation mechanism suited for field-deployable estimation of SCC in milk. In contrast, non-ionic surfactants like Tween-20 and Triton X-100 neither modify surface charge nor promote hydrophobic bridging, failing to induce aggregation. Further, CTAB, a cationic surfactant, did not promote aggregation as it may increase electrostatic repulsion at critical interaction sites.
The remarkable selectivity of our nanoparticle-based assay for somatic cells, in the presence of milk’s complex biological matrix, can be attributed to three factors. First, size exclusion plays a critical role. Our 8 nm IONP demonstrates optimal binding to somatic cells (10–15 μm in diameter; Fig. 2a). Second, positive charge matching ensures specific electrostatic interactions. The Af-IONP maintains a ζ-potential of +24 mV in milk buffer conditions (pH 6.5–6.7), which preferentially targets anionic phospholipids (e.g., phosphatidylserine, −15 to −20 mV) exposed on mammalian cell membranes66. In contrast, bacterial surfaces, while similarly anionic, present different charge distributions that prevent stable nanoparticle adhesion, as demonstrated by selectivity experiments (Fig. 2h). Third, membrane affinity is enhanced through hydrophobic interactions. The dodecyl chains of SDS (0.25%) facilitate nanoparticle anchoring to lipid rafts in somatic cell membranes, a feature absent in both casein micelles and bacterial cell walls. This mechanism possibly explains the assay’s robust performance in milk samples to detect milk SCC.
The development of the QuantM device marks a significant advancement in point-of-care diagnostics for SCC detection in milk, offering a portable, cost-effective, and efficient alternative to traditional methods. The device integrates several innovative features, including the MACC (Fig. 3a), AVM (Fig. 3b), and AVM reader (Fig. 3l) together enable the rapid detection and quantification of somatic cells with minimal technical expertise. This is of particular importance in low-resource settings, where access to advanced laboratory infrastructure may be limited. One of the novel aspects of the QuantM device is its ergonomic design and compact form factor, which make it highly portable and suitable for field applications. Unlike conventional diagnostic tools that require bulky, expensive equipment and specialized training, the QuantM device is designed to operate on a small footprint with low power consumption, running on battery power and utilizing 3D printing for customizable manufacturing. This makes the device ideal for rural or remote settings where power supply and technical infrastructure may be scarce. The ability to use the device in the field expands the accessibility of SCC detection, which is crucial for ensuring milk quality and safety, particularly in developing regions.
The AVM is a critical innovation in the QuantM device, specifically designed to facilitate the visualization and retention of nanoparticle-cell aggregates. The NCM incorporated into the AVM plays a pivotal role by ensuring fast fluid absorption and efficient capture of nanoparticle-cell complexes. The 0.8 µm NCM was specifically selected for its ability to retain these aggregates, as confirmed through the SEM image. This module allowed for rapid, on-site visualization of the aggregates, providing a real-time result that is particularly valuable in field settings. The development of this module significantly advances current methodologies that rely on slow and costly separation processes for somatic cell isolation.
Furthermore, the incorporation of an image-processing algorithm in the form of an AVM reader of the QuantM device enabled the transition from qualitative to quantitative somatic cell counting. By automating the image analysis process, the algorithm eliminates the need for manual assessment, ensuring greater accuracy, repeatability, and efficiency. The use of a computer vision (OpenCV) based machine learning linear regression model to process the images of nanoparticle-cell aggregates establishes quantification, a key advancement that significantly improves the precision of SCC measurements. This automated system enhances the usability of the device, making it accessible to users without specialized training.
The image processing algorithm has been integrated in Python-Flask67 to develop a web-based user interface (UI) in the AVM reader further enhancing its practicality. Through a seamless integration of hardware and software, the device allows users to capture, process, and analyze images directly on-site using a Raspberry Pi 4 integrated with a touchscreen display. The web application enables users to upload, crop, and process images for accurate quantification of somatic cell concentrations. The concave cleft on the surface of the AVM avoids the spread of aggregates and allows them to collect at the center. This design supports repeatability while capturing the ROI of the image. This easy-to-use interface ensures that the device is suitable for use in a wide range of settings, from dairy farms to remote healthcare clinics, where real-time analysis is crucial. Unlike conventional approaches that may require extensive equipment or prolonged incubation times, this method combines speed, specificity, simplicity, and high sensitivity, making it particularly well-suited for field applications and resource-limited settings.
The performance of the QuantM device was rigorously validated against flow cytometry, the gold-standard method for somatic cell counting. Flow cytometry was selected as the reference due to its high accuracy, sensitivity, and standardization in regulatory frameworks (e.g., ISO 13366-2). This technique employs DNA-specific fluorescent dyes (e.g., propidium iodide/thiazole orange, PI/TO) to selectively label intact somatic cells while excluding debris, enabling precise quantification even at low cell concentrations68. Unlike indirect methods (e.g., electrical conductivity or CMT), flow cytometry provides absolute cell counts, making it ideal for benchmarking novel technologies. The strong correlation (>90%) between QuantM and flow cytometry (Fig. 4d) confirms that our device achieves laboratory-grade accuracy while addressing key limitations of flow cytometry: high cost (~$2.1/sample), need for centralized labs, and technical expertise (Table 1, Supplementary Fig. 8). Field trials with 60 milk samples showed one false positive and two false negative results (Supplementary Fig. 6). The sensitivity, specificity, and accuracy and Bland-Altman analysis revealed minimal bias (mean bias: 0.00092), with 95% of differences falling within narrow limits of agreement (Fig. 4e). This alignment underscores QuantM’s suitability for field use without sacrificing precision. While traditional SCC detection relies on flow cytometry or Fossomatic® systems, these methods remain impractical for decentralized dairy sectors. Flow cytometry’s role in this study was pivotal as it provided an unambiguous, standardized metric to validate QuantM’s performance under real-world conditions (60 clinical samples; Supplementary Fig. 6). By demonstrating >90% sensitivity/specificity relative to flow cytometry, QuantM bridges the gap between laboratory accuracy and field deployability—a critical advancement for resource-limited settings.
The QuantM system represents a paradigm shift in somatic cell counting by overcoming critical limitations of existing technologies through three synergistic innovations. First, its novel nanoparticle-mediated detection mechanism fundamentally differs from conventional approaches: unlike flow cytometry, which requires fluorescent labeling (adding $1.50–2.00 per test in reagents) or electrical conductivity methods susceptible to milk composition variations. QuantM’s integrated sample processing addresses a major bottleneck in current workflows. Where systems like Fossomatic™ require separate centrifugation (15 min, 1000 × g) and Delaval cell counters need staining steps (5–10 min incubation), QuantM combines magnetic capture, SDS-mediated aggregation, and membrane filtration into a unified 5-minute workflow using just 1 mL of raw milk (Supplementary Movie 1). This integration reduces hands-on time by 4× compared to portable microscopy systems (Lactoscan SCC), while eliminating the need for sample pre-treatment–a critical advantage given that many field-level Indian dairy cooperatives lack centrifuges (NDDB 2023 report). QuantM reduces operational costs by 10-fold compared to flow cytometry while eliminating infrastructure requirements–a critical advantage for developing regions where 72% of collection centers lack reliable power (NDDB, 2023).
This affordability (Supplementary Table 1 and 2), combined with the device’s portability and battery-operated design, positions the QuantM device as an ideal tool for use in regions where financial constraints and access to reliable diagnostic infrastructure are significant barriers to public health improvements. The clinical evaluation of the device conducted with milk samples collected from dairy farms further highlights its robustness in field-level deployment. The ability of the device to produce high-quality, field-deployable results with minimal technical expertise required is a key advantage over conventional laboratory methods, which often involve complex workflows and specialized equipment. Usability testing with various user groups, including farmers, technicians, and veterinarians, showed no significant variation in performance among users (Supplementary Fig. 8).
The QuantM device addresses a critical gap in diagnostic capabilities for somatic cell detection in milk, directly supporting AMR mitigation efforts. By enabling early mastitis detection, QuantM reduces reliance on empiric antibiotics—a key driver of AMR in dairy systems20. This aligns with the WHO’s One Health approach to combat AMR through targeted interventions in animal husbandry6,69. Its proven performance in clinical validation, coupled with its affordability and ease of use, suggests that it has the potential to improve milk quality monitoring on a global scale, particularly in low-resource settings. Further research and deployment of the device in diverse field settings will be essential to assess its long-term effectiveness and scalability, but the promising results from this study suggest that the QuantM device could revolutionize somatic cell detection and enhance food safety in dairy production.
While the current system effectively quantifies somatic cells, it does not provide a complete diagnosis of mastitis or identify specific pathogens responsible for increased SCC levels. Additional research is needed to expand the system’s capabilities, such as integrating pathogen-specific biomarkers or testing for antibiotic residues in milk. Future work could focus on extending the device’s application for detecting other cell types or pathogens in milk, as well as integrating the technology with mobile or cloud-based data analysis systems for real-time health monitoring and decision support in dairy farms. The QuantM represents a significant advancement in milk quality assessment, contributing to better public health outcomes and safer milk production practices.
Methods
Material
(3-Aminopropyl)triethoxysilane (APTES), Acetic acid, acetone, bovine serum albumin, cetyltrimethylammonium bromide (CTAB), epoxy embedding medium kit (Cat no. 45359), glutaraldehyde (50%), hydrochloric acid (HCl), Iron sulfate (FeSO4.7H2O), led citrate, propidium iodide (PI), propylene oxide, osmium tetroxide, sodium acetate, sodium bicarbonate, sodium carbonate, sodium citrate, sodium dodecyl sulfate (SDS), sodium hydroxide, sodium phosphate monobasic, sodium phosphate dibasic, saponin, tween-20, triton X-100, uranyl acetate, were purchased from Sigma-Aldrich, USA. Liquid ammonia (25%) (Fisher Scientific), nitrocellulose membrane, and absorbent pads were procured from MDI membrane technologies, India. Neodymium bar magnet, eosin methylene blue agar (M317, Himedia), gelatin mannitol salt agar (M521, Himedia). Raspberry Pi-4 model 4 with 4GB RAM, CMOS camera with LED (480 p, 30 fps), LCD touch screen display. Poly lactic acid (PLA) fillament.
Synthesis and functionalization of IONP
IONP were synthesized using the reverse precipitation method70. A 14% ammonia solution (50 mL) was added dropwise to 0.4 N FeSO4.7H2O solution (100 mL) under continuous stirring at 600 rpm at room temperature until the formation of a black precipitate. The precipitate was washed with deionized water, using a strong magnet until the pH reached to 7.0. The prepared nanoparticles were then stored at 4 °C until further use.
IONP were functionalized with amine groups by dropwise addition of (3-Aminopropyl) triethoxysilane (APTES) to the nanoparticle suspension in a 2:1 volumetric ratio. Methanol (10 mL) was added, and the mixture was sonicated for 30 minutes at 40% amplitude with 15-second ‘ON’ and 30-second ‘OFF’ cycles. This was followed by the addition of glycerol up to the final concentration of 60% and kept for mechanical agitation at 90 °C for 6 hours. The Af-IONP were washed several times with deionized water using a strong magnet to remove residual glycerol, and stored at 4°C until use.
Size estimation of IONP by transmission electron microscopy (TEM)
IONP (0.01 mg/mL; 10 µL) were deposited onto a carbon-coated copper grid and dried overnight in a desiccator. TEM images were obtained using a JEOL 1400HR electron microscope equipped with an EMISS QUEMESA 11MP CCD camera. The nanoparticle diameters were measured using ImageJ software, and a particle size distribution curve was plotted based on the percentage frequency of the measured sizes.
X-ray diffraction
The phase composition of IONP was analyzed using a Malvern Panalytical X’pert Pro X-ray diffractometer with Cu Kα radiation (λ = 1.5406 Å). The samples were washed twice with acetone using a strong magnet to remove residual water and resuspended in acetone. A glass slide with a magnet underneath was used to hold the sample, and after complete acetone evaporation, the dried sample was subjected to XRD analysis. Data was collected with a step size of 0.008° and a dwell time of 47 seconds per step. The resulting diffractogram was compared against the Joint Committee on Powder Diffraction Standards (JCPDS) database, and Rietveld refinement was performed using X’Pert HighScore software71 to accurately determine peak positions and widths. Crystallite size was calculated using the Scherrer formula:
$${rm{tau }}=frac{Klambda }{beta cos {rm{theta }}}$$
(1)
where τ is the crystallite size, K is the shape factor, λ is the X-ray wavelength (λ = 1.54064 Å), β is the full width at half maximum, and θ is the diffraction angle.
FT-IR spectroscopy
FT-IR spectrum of powdered Af-IONP was obtained using Attenuated total reflectance (ATR) FT-IR (Thermo Scientific iD7 ATR NICOLET iS5) as described by ref. 72. The background spectrum was collected and the IONP (10 mg) were placed on a single-reflection diamond crystal and secured with a torque knob. The spectrum of the amine-functionalized nanoparticles was recorded over a wavenumber range of 400 to 4000 cm⁻¹ for 32 scans, using OMINC software. The spectrum was subjected to baseline correction for accurate assignment of peak positions.
Zeta potential measurement
The Af-IONP were sonicated at 40% amplitude in 10-second ‘ON’ and 10-second ‘OFF’ cycles for 15 minutes, either alone or in the presence of cells, to measure the changes in surface charge using the Anton Paar Litesizer™ 500. The zeta potential was recorded at different pH levels upon suspension of amine-functionalized IONP in respective buffers.
Vibrating sample magnetometry
IONP (10 mg) were placed in a magnetometer (Lakeshore 7404, Lake Shore Cryotronics, Westerville, OH, USA) under varying magnetic fields from −10 to +10 k Oersted in 200 Oersted at room temperature. Saturation magnetization, remanence, and coercivity were measured to assess the magnetic properties of the nanoparticles as described elsewhere73.
Sample preparation for TEM analysis of cells with Af-IONP
Mammary epithelial cells (HC11; 0.1 million cells) were suspended in phosphate-buffered saline (PBS, 10 mM; 1 mL) and incubated with Af-IONP (250 µg/mL) for 1 hour at room temperature. Cells were then fixed with 2.5% glutaraldehyde in 10 mM PBS for 2 hours at room temperature. Following fixation, cell-nanoparticle complexes were washed and incubated in 1% osmium tetroxide overnight in the dark.
The complexes were subsequently dehydrated through a graded acetone series (20%, 40%, 60%, 80%, and 100%), with each step lasting 30 minutes. After dehydration, the samples were treated with a 1:1 mixture of propylene oxide and epoxy medium at room temperature for 4 hours, followed by centrifugation at 9000 × g for 10 minutes. The resulting pellet was embedded in epoxy medium and cured at 60 °C for 48 hours. Ultra-thin sections (30–100 nm) were cut from the epoxy-embedded blocks, placed on copper grids, and stained with uranyl acetate and lead citrate to enhance contrast for TEM as described previously.
Somatic cell sequestration by Af-IONP and visualization
The optimal concentration of Af-IONP for effective cell sequestration was determined by testing varying cell numbers and nanoparticle concentrations. The cell-nanoparticle mixtures were incubated for 5 minutes, followed by magnetic separation using a strong magnet to isolate the cell–Af-IONP complexes. The protein content in the supernatant, reflecting the presence of cells, was quantified with the bicinchoninic acid assay (Thermo Scientific, Waltham, MA, USA).
At the optimized cell and Af-IONP concentrations, incubation time was further refined by measuring the protein content in the supernatant after magnetic separation at various intervals (<1, 1, 2, 5, 10, and 20 minutes). To evaluate aggregate formation, the cell–Af-IONP complexes were treated with surfactants such as Tween-20, Triton X-100, SDS, CTAB and saponin at various concentrations to note the ability to form visible aggregates. The black colored aggregate area was quantified by densitometry (image J – Java 1.8.0_345 64 bit). The aggregation causative factor in milk was further investigated by independently separating the cells through centrifugation and filtration. Milk samples that were positive in the aggregation assay were centrifuged at 1000 × g for 10 min to separate cellular (pellet) and non-cellular (supernatant) components. The pellet was resuspended in PBS. In another experiment, the milk was passed through a 0.2 µm membrane filter to isolate particulate (retentate) and soluble (flow-through) fractions.
Design and 3D Printing of the MACC and AVM
MACC and AVM were designed using Solid works (Version 33.2.0.0128) 3D CAD software, with user-friendly features to facilitate efficient sample processing and visualization. PrusaSlicer (Version 2.9.0) was used to generate support structures for overhang regions and to export the g-code file for 3D printing on Prusa i3 MK3S+ (PRUSA Research, Praque, Czech Republic) with polylactic acid (PLA) filament.
The MACC was created as a cylindrical device with a circular base (8.0 cm height × 6.5 cm diameter) having a semi-rotatory sample holder (4.5 cm height × 4.0 cm diameter) designed to process two samples simultaneously. A central axis enables clockwise and counterclockwise rotation of the sample holder. Diagonal slots on the base accommodate two bar magnets (2.0 cm height × 0.5 cm width) to allow magnetic exposure, with perpendicular windows. The sample holder has cylindrical extrusions (1.1 cm height × 3.8 cm diameter) to securely position two sample tubes.
Scanning electron microscopy (SEM)
The NCMs were air-dried at 65 °C for 1 hour, mounted onto stubs, and coated with a gold-palladium layer using a sputter coater (Polaron SC 7620, Quorum Technologies, Sussex, UK). Images were collected by SEM to assess aggregate retention, using a Carl Zeiss Evo18 (Jena, Germany) operating at 10 kV. Images were captured at ×3000 and ×5000 magnification.
Design and printing of AVM reader
The AVM reader comprises three main compartments: a 7-inch touchscreen display, memory (Raspberry Pi 4 motherboard with 8 GB RAM), and a capture compartment housing a CMOS camera. The capture module (3.5 × 3.8 × 6.0 cm) was 3D printed in white filament and securely mounted within the capture compartment to facilitate image acquisition of the AVM. This module integrates a CMOS camera equipped with light-emitting diodes positioned opposite the AVM slot, customized with a 10.0 mm focal length, 39.0 mm focus distance, and a 90° field of view.
A rectangular opening (2.5 × 0.62 cm) on the front of the AVM reader aligns the AVM precisely with the camera module. This alignment allows the AVM to slide on a track at the base of the camera module, ensuring consistent positioning. The display was mounted securely in its designated slot, and the camera module connected to the Raspberry Pi motherboard. Additional slots for a power switch and socket were incorporated on the back of the AVM reader.
AVM reader software
An image processing algorithm was developed in text editor (Sublime Text editor, © Sublime HQ Pty Ltd, Woollahra, Sydney) on a Windows 11 operating system. The software has been installed in AVM reader, which was equipped with a Samsung Class 4 micro-SD card preloaded with the official Raspberry Pi NOOBS package-Raspbian operating system.
QuantM (patent application number: 201941044822, Dt: 05-11-2019)
1 mL of milk was added to a sample tube placed in the MACC device. Then, two drops of IONP (which is equivalent to 500 µg) were added, mixed well, facing the window (blue) side and incubated for 5 minutes, allowing interaction of IONP with somatic cells in the milk. After that, the sample was rotated towards the magnet side, which is away (yellow) from the window, for sequestration of the cell-IONP complex. Subsequently, four drops of SDS (0.25%) were added for aggregation formation. After that, the formed aggregates were transferred to the surface of the nitrocellulose membrane of AVM, for quantification with the AVM reader.
Image processing
The image processing algorithm was trained using six carefully constructed datasets representing the full physiological range of somatic cell concentrations (0–0.2 million cells/mL). We created six stratified concentrations (0, 0.01, 0.02, 0.05, 0.1, and 0.2 million cells/mL) through serial dilution in cell-free milk collected from healthy animals. Each dataset’s images were captured under standardized conditions using the QuantM device for analysis. The experimental observations were verified again by flow cytometry (Fossomatic™ 7 DC) and direct microscopic (Petroff-Hausser chamber) counting methods.
Flow cytometry
Freshly collected milk (10 mL) was centrifuged at 400 × g for 10 minutes to separate the components. The fat layer was carefully removed with a spatula, and the supernatant was discarded. The resulting pellet was washed three times with PBS (10 mM) containing Triton X-100 (0.01%), centrifuging each time at 400 × g for 10 minutes. The final pellet was then resuspended in 1 mL of PBS (10 mM). Liquid counting beads, TO, and PI were utilized according to the manufacturer’s protocol (Cell Viability Kit, BD Biosciences, CA).
Somatic cell counting in a clinical sample
Milk samples were transported to the laboratory under cold conditions. Upon arrival, somatic cell counts were analyzed using a QuantM. Additionally, SCC were processed using flow cytometry. The SCC obtained from the AVM reader was compared with that from flow cytometry to assess sensitivity, specificity, accuracy, Bland-Altman, and Spearman correlation.
Data representation and Statistical analysis
All experiments were performed in triplicate, and the data are presented as mean ± standard error of the mean. A power analysis was conducted to estimate the sample size necessary to distinguish between comparison groups. Statistical validation was performed using one-way analysis of variance, followed by the Tukey post-hoc test for significance. The Bland-Altman analysis and Spearman correlation coefficient were calculated at a 95% confidence interval to assess the strength and direction of the association between variables.
Data availability
All datasets generated or analyzed during this study are available from the corresponding author upon reasonable request. The source code used in this work is openly accessible via the following GitHub repository: https://github.com/Yathi123/QuantM.
References
-
Dincer, C. et al. Disposable sensors in diagnostics, food, and environmental monitoring. Adv. Mater. 31, 1806739 (2019).
-
Ding, T. et al. A multiplex RT-PCR assay for S. aureus, L. monocytogenes, and Salmonella spp. detection in raw milk with pre-enrichment. Front. Microbiol. 8, 989 (2017).
-
Heidinger, J. C., Winter, C. K. & Cullor, J. S. Quantitative microbial risk assessment for Staphylococcus aureus and Staphylococcus enterotoxin A in raw milk. J. Food. Prot. 72, 1641–1653 (2009).
-
Kerro Dego, O. & Vidlund, J. Staphylococcal mastitis in dairy cows. Front. Vet. Sci. 11, 1356259 (2024).
-
Yadav, J. et al. Comparative evaluation of pathogenic bacterial incidence in raw and pasteurized milk. Int. J. Eng. Sci 3, 11–20 (2014).
-
World Health Organization. General information related to microbiological risks in food. http://www.who.int/foodsafety/micro/general/en/index.html (2012).
-
Alhussien, M. N. & Dang, A. K. Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: an overview. Vet. world 11, 562 (2018).
-
Angelopoulou, A. et al. Somatic cell count as an indicator of subclinical mastitis and increased inflammatory response in asymptomatic lactating women. Microbiol. Spectr. 12, e04051–23 (2024).
-
EFSA Panel on Biological Hazards (BIOHAZ) Scientific opinion on the public health risks related to the consumption of raw drinking milk. EFSA J. 13, 3940 (2015).
-
Gillespie, B. E. et al. Evaluation of bulk tank milk microbiological quality of nine dairy farms in Tennessee. J. Dairy Sci. 95, 4275–4279 (2012).
-
Hayes, M. C. & Boor, K. Raw milk and: fluid milk products. In Applied dairy microbiology (pp. 79–96). CRC Press (2001).
-
Lejeune, J. T. & Rajala-Schultz, P. J. Food safety: unpasteurized milk: a continued public health threat. Clin. Infect. Dis. 48, 93–100 (2009).
-
Newell, D. G. et al. Food-borne diseases—the challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 139, S3–S15 (2010).
-
Sebastianski, M., Bridger, N. A., Featherstone, R. M. & Robinson, J. L. Disease outbreaks linked to pasteurized and unpasteurized dairy products in Canada and the United States: a systematic review. Canadian. J. Public Health 113, 569–578 (2022).
-
Tadesse, H. A. et al. Antimicrobial resistance profile of E. coli isolated from raw cow milk and fresh fruit juice in Mekelle, Tigray, Ethiopia. Vet. Med. Int. 2018, 8903142 (2018).
-
Ulusoy, B. H. & Chirkena, K. Two perspectives of Listeria monocytogenes hazards in dairy products: the prevalence and the antibiotic resistance. Food Qual. Saf. 3, 233–241 (2019).
-
Wilson, D. J., Gonzalez, R. N. & Das, H. H. Bovine mastitis pathogens in New York and Pennsylvania: prevalence and effects on somatic cell count and milk production. J. Dairy Sci. 80, 2592–2598 (1997).
-
Koski, L. et al. Foodborne illness outbreaks linked to unpasteurised milk and relationship to changes in state laws–United States, 1998–2018. Epidemiol. Infect. 150, e183 (2022).
-
Mungai, E. A., Behravesh, C. B. & Gould, L. H. Increased outbreaks associated with nonpasteurized milk, United States, 2007-2012. Emerg. Infect. Dis. 21, 119–122 (2015).
-
Oliver, S. P., Boor, K. J., Murphy, S. C. & Murinda, S. E. Food safety hazards associated with consumption of raw milk. Foodborne Pathog. Dis. 6, 793–806 (2009).
-
Robinson, T. J., Scheftel, J. M. & Smith, K. E. Raw milk consumption among patients with non-outbreak-related enteric infections, Minnesota, USA, 2001-2010. Emerg. Infect. Dis. 20, 38–44 (2014).
-
Sahoo, S., Behera, M. R., Mishra, B., Sahoo, P. & Kar, S. Antibiotic-resistant bacteria in bovine milk in India. J. Adv. Vet. Anim. Res 10, 21 (2023).
-
The dangers of raw milk: unpasteurized milk can pose a serious health risk. https://www.fda.gov/food/buy-store-serve-safe-food/dangers-raw-milk-unpasteurized-milk-can-pose-serious-health-risk (2024).
-
Yamin, D. et al. Current and future technologies for the detection of antibiotic-resistant bacteria. Diagnostics 13, 3246 (2023).
-
Sauerborn, E. et al. Detection of hidden antibiotic resistance through real-time genomics. Nat. Commun. 15, 5494 (2024).
-
EFSA, E. The European Union one health 2021 zoonoses report. EFSA J 20, 1–273 (2022).
-
Tam, C., Larose, T., & O’Brien, S. J. Costed extension to the Second Study of Infectious Intestinal Disease in the Community: Identifying the proportion of foodborne disease in the UK and attributing foodborne disease by food commodity. Food Standards Agency (2014).
-
Angulo, F. J., LeJeune, J. T. & Rajala-Schultz, P. J. Unpasteurized milk: a continued public health threat. Clin. Infect. Dis. 48, 93–100 (2009).
-
D’amico, D. J. Microbiological quality and safety issues in cheesemaking. Microbiol. Spectr. 251–309 (2014).
-
Ruegg, P. L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci 100, 10381–10397 (2017).
-
Bezerra, J. D. S. et al. Effect of high somatic cell counts on the sensory acceptance and consumption intent of pasteurized milk and coalho cheese. Food Sci. Technol. 41, 423–431 (2020). (suppl 2).
-
Fonseca, M. et al. Usefulness of differential somatic cell count for udder health monitoring: Association of differential somatic cell count and somatic cell score with quarter-level milk yield and milk components. J. Dairy Sci. 108, 3900–3916 (2025).
-
Leitner, G., Krifucks, O., Merin, U., Lavi, Y. & Silanikove, N. Interactions between bacteria type, proteolysis of casein and physico-chemical properties of bovine milk. Int. Dairy J. 16, 648–654 (2006).
-
Li, N., Richoux, R., Boutinaud, M., Martin, P. & Gagnaire, V. Role of somatic cells on dairy processes and products: a review. Dairy Sci. Technol. 94, 517–538 (2014).
-
Miglior, F. et al. Genetic analysis of milk urea nitrogen and lactose and their relationships with other production traits in Canadian Holstein cattle. J. Dairy Sci. 90, 2468–2479 (2007).
-
Antanaitis, R., Juozaitienė, V., Jonike, V., Baumgartner, W. & Paulauskas, A. Milk lactose as a biomarker of subclinical mastitis in dairy cows. Animals 11, 1736 (2021).
-
Berglund, I., Pettersson, G., Östensson, K. & Svennersten‐Sjaunja, K. Quarter milking for improved detection of increased SCC. Reprod. Domest. Anim. 42, 427–432 (2007).
-
Kocabaş, D. S., Lyne, J. & Ustunol, Z. Hydrolytic enzymes in the dairy industry: applications, market and future perspectives. Trends Food Sci. Technol 119, 467–475 (2022).
-
Ma, Y. et al. Effects of somatic cell count on quality and shelf-life of pasteurized fluid milk. J. Dairy Sci. 83, 264–274 (2000).
-
Wang, W., Sun, B., Deng, J. & Ai, N. Addressing flavor challenges in reduced-fat dairy products: a review from the perspective of flavor compounds and their improvement strategies. Food Res. Int. 118, 14478 (2024).
-
Hisira, V. et al. Comparative analysis of methods for somatic cell counting in cow’s milk and relationship between somatic cell count and occurrence of intramammary bacteria. Vet. Sci. 10, 468 (2023).
-
Jacobsen, L. A., Niesen, A. M., Lucey, P. & Rossow, H. A. Evaluation of cow-side meters to determine somatic cell count in individual cow quarter and bulk-tank milk samples. Animals 13, 2169 (2023).
-
Pereira, A. R., Oliveira, C. A., Marques, D. C., Machado, P. F. & Sarriés, G. A. Correlation between milk somatic cell counting of samples prepared with two detergents, and analyzed by the Somacount. Arq. Bras. Med. Vet. Zootec. 52, 218–219 (2000).
-
Press Information Bureau. Ministry of cooperation: white revolution 2.0 under azadi ka amrit mahotsav (2025).
-
Rasheed, S., ul Haq, M. A., Ahmad, N. & Hussain, D. Smartphone-integrated colorimetric and microfluidic paper-based analytical devices for the trace-level detection of permethrin. Food Chem 429, 136925 (2023).
-
Sun, X. et al. Milk somatic cell count: From conventional microscope method to new biosensor-based method. Trends Food Sci. Technol. 135, 102–114 (2023).
-
Phiphattanaphiphop, C., Leksakul, K., Nakkiew, W., Phatthanakun, R. & Khamlor, T. Fabrication of spectroscopic microfluidic chips for mastitis detection in raw milk. Sci. Rep. 13, 6041 (2023).
-
Moon, J. S. et al. Application of a new portable microscopic somatic cell counter with disposable plastic chip for milk analysis. J. Dairy Sci. 90, 2253–2259 (2007).
-
Carminati, M., Ferrari, G., Vahey, M. D., Voldman, J. & Sampietro, M. Miniaturized impedance flow cytometer: design rules and integrated readout. IEEE Trans. Biomed. Circuits Syst. 11, 1438–1449 (2017).
-
Fadillah, A. et al. Evaluation of factors associated with bulk milk somatic cell count and total plate count in Indonesian smallholder dairy farms. Front. Vet. Sci. 10, 1280264 (2023).
-
He, L. et al. A sample-preparation-free, point-of-care testing system for in situ detection of bovine mastitis. Anal. Bioanal. Chem. 415, 5499–5509 (2023).
-
Ramuada, M., Tyasi, T. L., Gumede, L. & Chitura, T. A practical guide to diagnosing bovine mastitis: a review. Front. Anim. Sci. 5, 1504873 (2024).
-
Mohammed, I., Al Shehri, D., Mahmoud, M., Kamal, M. S. & Alade, O. S. A surface charge approach to investigating the influence of oil contacting clay minerals on wettability alteration. ACS omega 6, 12841–12852 (2021).
-
Park, Y. et al. Stability of superparamagnetic iron oxide nanoparticles at different pH values: experimental and theoretical analysis. Langmuir 28, 6246–6255 (2012).
-
Rios, A., Zougagh, M. & Bouri, M. Magnetic (nano) materials as a useful tool for sample preparation in analytical methods. A review. Anal. Methods 5, 4558–4573 (2013).
-
Woo, S. et al. Charge-modulated synthesis of highly stable iron oxide nanoparticles for in vitro and in vivo toxicity evaluation. Nanomaterials 11, 3068 (2021).
-
Tian, W. et al. Efficient capture and T2 magnetic resonance assay of Candida albicans with inorganic nanoparticles: role of nanoparticle surface charge and fungal cell wall. ACS Biomater. Sci. Eng. 5, 3270–3278 (2019).
-
Gu, H., Ho, P. L., Tsang, K. W., Wang, L. & Xu, B. Using biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram-positive bacteria at ultralow concentration. J. Am. Chem. Soc 125, 15702–15703 (2003).
-
Huang, Y. F., Wang, Y. F. & Yan, X. P. Amine-functionalized magnetic nanoparticles for rapid capture and removal of bacterial pathogens. Environ. Sci. Technol. 44, 7908–7913 (2010).
-
Kadam, R., Maas, M. & Rezwan, K. Selective, agglomerate-free separation of bacteria using biofunctionalized, magnetic janus nanoparticles. ACS Appl. Bio Mater 2, 3520–3531 (2019).
-
Li, Z., Ma, J., Ruan, J. & Zhuang, X. Using positively charged magnetic nanoparticles to capture bacteria at ultralow concentration. Nanoscale Res. Lett. 14, 1–8 (2019).
-
Martínez-Matamoros, D. et al. Preparation of functionalized magnetic nanoparticles conjugated with feroxamine and their evaluation for pathogen detection. RSC adv. 9, 13533–13542 (2019).
-
Szilagyi, I., Trefalt, G., Tiraferri, A., Maroni, P. & Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 10, 2479–2502 (2014).
-
Zhang, Y. & Zhou, N. Electrochemical biosensors based on micro‐fabricated devices for point‐of‐care testing: a review. Electroanalysis 34, 168–183 (2022).
-
Zhang, W., Crittenden, J., Li, K. & Chen, Y. Attachment efficiency of nanoparticle aggregation in aqueous dispersions: modeling and experimental validation. Environ. Sci. Technol. 46, 7054–7062 (2012).
-
Loufakis, D. N., Cao, Z., Ma, S., Mittelman, D. & Lu, C. Focusing of mammalian cells under an ultrahigh pH gradient created by unidirectional electropulsation in a confined microchamber. Chem. Sci. 5, 3331–3337 (2014).
-
Sharma M., Khan, M. S., & Singh, J. Python & Django the fastest growing web development technology. In 2024 IEEE 1st Karachi Section Humanitarian Technology Conference (KHI-HTC) (1-9). IEEE (2024).
-
Zecconi, A. et al. Differential somatic cell count as a marker for changes of milk composition in cows with very low somatic cell count. Animals 10, 604 (2020).
-
Sharma, G. et al. Comparing the effectiveness of different approaches to raise awareness about antimicrobial resistance in farmers and veterinarians of India. Front. Public Health 10, 837594 (2022).
-
Zawrah, M. F., El Shereefy, E. S. E. & Khudir, A. Y. Reverse precipitation synthesis of ≤10 nm magnetite nanoparticles and their application for removal of heavy metals from water. Silicon 11, 85–104 (2019).
-
Escoda-Torroella, M., Moya, C., Rodríguez, A. F., Batlle, X. & Labarta, A. Selective control over the morphology and the oxidation state of iron oxide nanoparticles. Langmuir 37, 35–45 (2020).
-
Querido, W., Ailavajhala, R., Padalkar, M. & Pleshko, N. Validated approaches for quantification of bone mineral crystallinity using transmission Fourier transform infrared (FT-IR), attenuated total reflection (ATR) FT-IR, and Raman spectroscopy. Appl. Spectrosc. 72, 1581–1593 (2018).
-
Keshta, B. E., Gemeay, A. H. & Khamis, A. A. Impacts of horseradish peroxidase immobilization onto functionalized superparamagnetic iron oxide nanoparticles as a biocatalyst for dye degradation. Environ. Sci. Pollut. Res. 29, 6633–6645 (2021).
-
Gonzalo, C., Linage, B., Carriedo, J. A. & De La Fuente, L. F. Short communication: evaluation of the overall accuracy of the DeLaval cell counter for somatic cell count in ovine milk: effect of soak time in diluted and undiluted milk samples. J. Dairy Sci. 91, 3114–3118 (2008).
-
Kawai, K. et al. Reliability in somatic cell count measurement of clinical mastitis milk using D e L aval cell counter. Anim. Sci. J. 84, 805–807 (2013).
-
Zigo, F. et al. Impact of humic acid as an organic additive on the milk parameters and occurrence of mastitis in dairy cows. Slovak J. Food. Sci./Potr., 14, 358–364 (2020).
-
Bonestroo, J. et al. Forecasting chronic mastitis using automatic milking system sensor data and gradient-boosting classifiers. Comput. Electron. Agric. 198, 107002 (2022).
-
Khatun, M., Bruckmaier, R. M., Thomson, P. C., House, J. & García, S. C. Suitability of somatic cell count, electrical conductivity, and lactate dehydrogenase activity in foremilk before versus after alveolar milk ejection for mastitis detection. J. Dairy Sci. 102, 9200–9212 (2019).
-
O’Mahony, M. C. et al. Milk amyloid A: Correlation with cellular indices of mammary inflammation in cows with normal and raised serum amyloid A. Res. Vet. Sci. 80, 155–161 (2006).
-
Taghdiri, M., Karim, G., Safi, S., Rahimi Foroushani, A. & Motalebi, A. Study on the accuracy of milk amyloid A test and other diagnostic methods for identification of milk quality. Vet. Res. Forum. Int. Q. J 9, 179–185 (2018).
Acknowledgements
We would like to acknowledge the Department of Biotechnology (grant numbers BT/PR41122/ATGC/127/48/2020), Government of India, for funding the project, and National Institute of Animal Biotechnology (NIAB), Hyderabad, Government of India, for providing the infrastructure and laboratory space to carry out this work. Y.T. is thankful to the University Grants Commission (UGC), Government of India, for Junior and Senior Research Fellowships. Y.T. and P.K. would like to acknowledge the Manipal Academy of Higher Education (MAHE) for academic support. Muskan is thankful to the Department of Biotechnology, Government of India, for Senior Research Fellowships and the Regional Center for Biotechnology, Faridabad, for academic support. The authors extend their gratitude for the help rendered for the characterization of IONP from Dr. Anshu Gaur, School of Physics, University of Hyderabad. The authors further acknowledge Acrovet Animal Health LLP for providing CAD support.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tammineni, Y., Kumar, P., Reddy, S. et al. Portable and affordable device for on-site quantitative detection of somatic cells in milk. npj Sci Food 9, 182 (2025). https://doi.org/10.1038/s41538-025-00519-3
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41538-025-00519-3