Introduction
Pneumonia is a serious lower respiratory infectious disease caused by microbial pathogens such as viruses, bacteria, fungi, and pneumonia results in millions of deaths every year, ranking as the 6th leading cause of death in the world1,2,3,4. As important components of the lung immune system, alveolar macrophages (AMs) are mainly responsible for phagocytosis and clearance of microbes and other foreign matters entering the lungs. AMs can undergo polarization towards either an M1 or M2 phenotype. M1 macrophages predominantly promote proinflammatory processes essential for host defense, whereas M2 macrophages facilitate anti-inflammatory responses and tissue remodeling5,6,7. However, once AMs are unable to control the replication of microbial pathogens, the balance of macrophage polarization disrupts, and excessive M1 polarization and cytokines secretion further cause the cytokine storm8,9,10. Therefore, pathogens, macrophages and cytokines are the key factors causing the deterioration of pneumonia. As a natural plant compound, curcumin (Cur) with antioxidant, anti-inflammatory, and immune regulation properties has shown great potential in the prevention and treatment of tissue damage and inflammatory diseases11,12,13,14. Cur mainly exerts anti-inflammatory effects by inhibiting macrophage infiltration and regulating macrophage polarization. It can directly induce macrophage polarization into anti-inflammatory M2 phenotype and also promote the repolarization of existing M1 macrophage towards M2 phenotype15,16. Cur can reduce the expression of inflammatory factors by blocking signaling pathways, however it is unable to directly neutralize the inflammatory factors produced already in the lungs17,18. Strategy capable of macrophage regulation, and pathogen and cytokine clearance would be very attractive for efficient pneumonia therapy.
Biomimetic cell membrane-coated nanoparticles, owning to the reserved membrane proteins and membrane structure, possess special properties such as specific recognition, pathogens neutralization, and toxin adsorption19,20,21. Macrophage cell membrane is generally overexpressed with receptors specific for IL-1, IL-6 and other proinflammatory factors, enabling for the capture and neutralization of pro-inflammatory cytokines, which plays a crucial role in restoring the homeostasis of the inflammatory microenvironment22,23,24. By genetic engineering transformation, for example Angiotensin converting enzyme 2 (ACE2) introduction, the constructed cell membrane cloaking nanoparticles can simulate natural host cells to achieve efficient combination with SARS-CoV-2, thus blocking virus invasion and inhibiting the abnormal pulmonary inflammatory reaction25,26. However, cell membrane camouflage strategy is still lacking the ability of autonomous motion and controllable navigation27, which greatly hinders the efficient and effective pathogens and cytokines neutralization.
Micro-/nanorobots are active colloidal micro-/nanoparticles with capability of converting external energies into their own mechanical kinetics28,29. Due to their self-propulsive property, these miniaturized robots have attracted increasing research interests in various fields such as environmental remediation, sensing and biomedicine30,31,32. The autonomous motion of micro-/nanorobots is particularly prominent especially in confined spaces, for example, lungs27,33, joint cavities34,35, and gastrointestinal tract36,37,38,39, which can further increase the interaction between robots themselves and tissue microenvironment. Among micro-/nanorobots, biohybrid ones have been showing enormous potentials in many aspects owing to their superior biocompatibility, higher energy conversion efficiency and many other characteristics. During the design and fabrication of biohybrid micro-/nanorobots, natural cells or bacteria are generally utilized as the main components, which can significantly reduce the biological toxicity and immune responses40,41. By incorporating intact cells with multifunctionality, biohybrid micro-/nanorobots are capable of operating smartly and responding to the microenvironment16,42,43. Natural magnetotactic bacteria AMB-1 with good biosafety and no clinical infection has magnetosomes that are sensitive to external magnetic field, thus providing the ability to move along the magnetic line and be precisely controlled by rotating magnetic fields44. Therefore, AMB-1 can actively and accurately transport cargoes to specific region under magnetic manipulation, and achieve deep tissue penetration and retention based on motion enhancement.
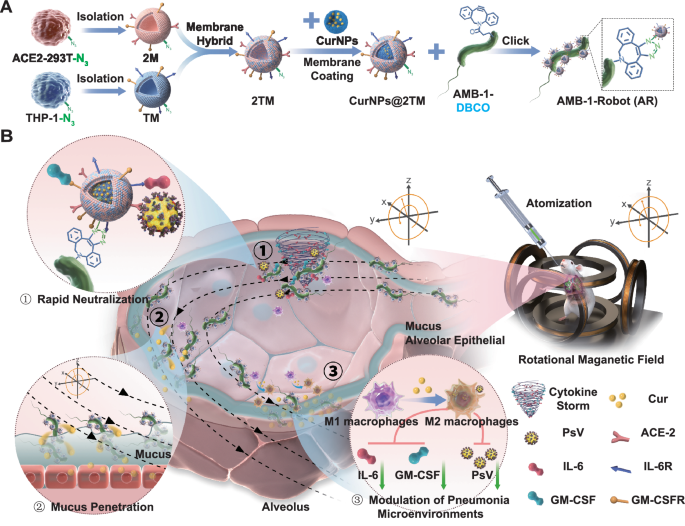
In this work, we develop a biomimetic microrobot system based on natural magnetotactic bacteria AMB-1 for active and efficient pneumonia therapy. THP-1 and ACE2-293T cells are first incubated with azido sugar Ac4GalNAz and the resulting hybrid cell membranes with N3 functionalization (2TM) are then fused onto Cur loaded human serum albumin (HSA) nanoparticles (CurNPs), followed by the conjugation with dibenzocyclooctyne (DBCO) modified AMB-1 via click chemistry, here referred as CurNPs@2TM-AMB-1 (AR) (Fig. 1A). Under the precise manipulation of an external rotating magnetic field (RMF), the developed AR are capable of swimming in a controlled manner and efficiently neutralizing inflammatory factors and SARS-CoV-2 pseudovirus (PsV) due to the reserved receptors from the cell membrane cloaking strategy, thereby blocking the virus invasion and reducing the damage of inflammatory factors to the lungs (Fig. 1B). Based on the magnetic actuation, active AR penetrate into the lung mucus layer and delivered the loaded Cur deeply, thus playing a significant role in pneumonia treatment and pneumonia microenvironment regulation.
A N3-labeling hybrid membrane nanoparticle CurNPs@2TM were attached to DBCO-labeling magnetic bacteria AMB-1 through click chemistry to form AR. B Schematic diagram of the application of AR in the treatment of pneumonia. After i.t. inhalation, the magnetic driven AR reach the alveoli, and under RMF, AR move and quickly neutralize inflammatory factors and PsV. Then, magnetically actuated AR penetrate the thick mucus layer to achieve efficient release and long-term retention of Cur. Under the regulation of Cur, the pneumonia microenvironment is alleviated, including the polarization of pro-inflammatory M1 macrophages into anti-inflammatory M2 macrophages. 2 M, TM, and 2TM refer to membranes derived from ACE2-293T cells, THP-1 cells, and their hybrid, respectively. DBCO is dibenzocyclooctyne. PsV denotes pseudovirus. IL-6 and GM-CSF are interleukin-6 and granulocyte-macrophage colony stimulating factor. ACE2 stands for angiotensin-converting enzyme 2.
Results
Fabrication of AR
Biomimetic N3-labeling cell membrane-coated nanoparticles (CurNPs@2TM) were first constructed. As shown in Fig. 2A, ACE2-293T cells and THP1 cells were pre-treated with N3 functionalized Ac4GalNAz respectively and the N3 functionalization was introduced via glycometabolism. To confirm the existence of N3 group, fluorescent DBCO-Cy5 was added into the cells and the click chemistry between DBCO and N3 was then conducted, followed by fluorescence microscopy imaging (Fig. 2B, C). Compared with the cells without any treatments, the cells pre-treated with Ac4GalNAz showed significant red fluorescence signal. Flow cytometry was further used to verify the successful introduction of N3 groups on the surface of THP1 cells and ACE2-293T cells (Fig. 2D–I and Supplementary Fig. 1). After N3 labeling, the resulting ACE2-293T cell membrane (2 M) and THP1 cell membrane (TM) were extracted and fused for the formation of 2M-TM hybrid cell membrane with N3 functionalization (2TM). Meanwhile, CurNPs were synthesized by assembling HSA and Cur under sonication. And 2TM was then coated onto the formed CurNPs by repeating extrusion to obtain CurNPs@2TM. 2 M was further labeled with DII, and TM was labeled with DID. Then the hybrid cell membrane with fluorescence labeling was coated onto CurNPs via repeating extrusion, followed by observation with inverted fluorescence microscope. The result showed that the fluorescence of 2 M and TM was mostly overlapped with that of Cur, indicating that CurNPs@2TM was indeed composed of the mixed cell membranes. (Supplementary Fig. 2). As shown in Fig. 2J, core-shell structure was clearly observed by transmission electron microscopy (TEM) with cell membrane thickness of about 25 nm. And the size of CurNPs@2TM with narrow distribution increased from 121 ± 3.05 to 161 ± 2.50 nm after hybrid membrane coating (Supplementary Fig. 3). The zeta potential of CurNPs@2TM decreased from −19.8 ± 0.40 to −29.0 ± 0.92 mV (Supplementary Fig. 4), indicative of successful cloaking with a negatively charged cell membrane. Western blot analysis demonstrated that CurNPs@2TM retained key membrane receptors involved in viral and cytokine neutralization, including ACE2 for SARS-CoV-2, CD116 for GM-CSF, and IL6ST for IL-6 (Fig. 2K and Supplementary Fig. 25), demonstrating the effective decoration of CurNPs with 2TM hybrid membranes.
A Schematic of the formation of CurNPs@2TM. B–C Fluorescence microscopy image of B, THP1 cells and C, ACE2-293T cells after pre-treating with Ac4GalNAz and clicking with DBCO-Cy5 (Cy5, red; cell nuclei, blue). D-G Flow cytometric profiles of D, E THP1 cells and F, G ACE2-293T cells with or without Ac4GalNAz incubation and DBCO-Cy5 modification. H Quantification of Cy5+ cells in Fig. D, E (n = 3 independent samples). I Quantification of Cy5+ cells in Fig. F, G (n = 3 independent samples). J TEM images of CurNPs and CurNPs@2TM. K Western blotting analysis of ACE2, IL-6 receptor IL6ST, and GM-CSF receptor CD116 in 2 M, TM, 2TM and CurNPs@2TM, respectively. ATP1A1 indicates Na+/K+-ATPase. L Schematic of the fabrication of AR via click chemistry. M Pseudo-colored SEM images of AMB-1 and AR. N Fluorescence microscopy image of AMB-1 and AR (CurNPs@2TM was observed in green based on Cur FL signal). HSA, DBCO, Cur, ACE2, and FL refer to human serum albumin, dibenzocyclooctyne, curcumin, angiotensin-converting enzyme 2, and fluorescence, respectively. Data in H-I are presented as mean ± S.D. Statistical significance was determined using two-tailed Student’s t-tests (****P < 0.0001). Experiments in B–G, J, K, M, and N were independently repeated three times with similar results, and a representative result is shown for each. Source data are provided as a Source Data file.
Natural magnetotactic bacteria AMB-1 were cultivated in enriched magnetic spirillum growth medium (EMSGM). In order to obtain DBCO modification, DBCO-PEG4-NHS ester was added into AMB-1 and esterification reaction was conducted for the formation of DBCO-labeled AMB-1 (Fig. 2L). CurNPs@2TM with N3 group was then reacted with DBCO-labeled AMB-1 via click chemistry, resulting in the successful fabrication of AR. Scanning electron microscopy (SEM) was further used to confirm the surface modification of AMB-1. As shown in Fig. 2M, AMB-1 with smooth surface was clearly observed. Whereas, several nanoparticles were covalently attached onto the surface of the bacteria after click reaction with CurNPs@2TM. Due to the inherent fluorescence of Cur, homogeneous and intense fluorescent signals were clearly visualized for AR, thus demonstrating that CurNPs@2TM were indeed firmly modified onto the AMB-1 surface after multiple washing steps (Fig. 2N). After CurNPs@2TM introduction, zeta potential of AR also showed a slight increase from −16.2 ± 0.55 to −18.5 ± 0.41 mV (Supplementary Fig. 4). Click chemistry has the advantage of mild reaction conditions, and it usually has little effect on cell viability when carried out on the cell surface. Only a few dead bacteria were observed after click chemistry for 1 day (Supplementary Fig. 5). The viability of bacteria was also evaluated. After click chemistry for 1 day, the numbers of AMB-1 and AR colonies were similar and the survival rate of AR was about 95% (Supplementary Fig. 6). In order to investigate the survival time of AMB-1 after being loaded with CurNPs@2TM, we studied the growth curve of AR and found that the growth of bacteria connected with CurNPs@2TM was slightly affected with no significant difference, indicating that magnetotactic bacteria can survive normally after loading with CurNPs@2TM (Supplementary Fig. 7). The physicochemical characteristics of AR were also investigated. The UV-vis and fluorescence spectroscopy revealed absorption and emission peaks of AR at 432 and 544 nm, respectively, in agreement with the spectral profile of CurNPs@2TM. (Supplementary Fig. 8–9). The effective FL intensity of the loaded Cur was also meaningful for FL imaging in vivo, which could be used to monitor the accumulation of AR.
Motion behavior of AR
After confirming the successful fabrication of AR, the motion behaviors of biomimetic magnetobacterial microrobots were further investigated in simulated lung fluid (SLF) under a homogeneous RMF generated by a homemade three-dimensional Helmholtz coil system. As illustrated in Fig. 3A, the applied RMF allows AR to translate a rotational motion into translational propulsion. As a magnetotactic bacterium, AMB-1 with a diameter of 0.5 µm and a length of 3 µm has intracellular magnetosomes with an average size of 50 nm, which are arranged in chains along the motion axis of the bacteria consistent with the previous report (Fig. 3B)45,46, providing the prerequisite for magnetic actuation. The magnetic properties of AMB-1 and AR were also evaluated by a vibrating sample magnetometer (VSM). The magnetic hysteresis loops showed that both AMB-1 and AR were superparamagnetic, and the saturation magnetization (Ms) of AMB-1 and AR was 1.82 emu/g and 1.71 emu/g respectively (Fig. 3C), indicating that the superparamagnetic property of AMB-1 was reserved after CurNPs@2TM introduction.
A Schematic of magnetic actuation of AR, wherein V denotes the forward velocity, B represents the strength of the magnetic field, f denotes the frequency of the magnetic field, ω represents the rotation velocity of AR, and θ indicates the tilt angle between z and rotation axis of AR. B TEM images of AMB-1. The magnified view highlights the magnetosome chains within AMB-1 (right panel). The experiment was repeated independently three times with similar results, and a representative result is shown. C Magnetic hysteresis loops of AMB-1 and AR. The experiment was repeated independently three times with similar results, and a representative result is shown. D Velocity of AMB-1 and AR in SLF under magnetic field with different frequencies (n = 20 independent samples). E Representative tracking trajectories of AMB-1 in SLF for 20 s. F Parabola fitting of MSD obtained from the optical tracking of AMB-1 in different frequencies. G Representative tracking trajectories of AR in SLF for 20 s. H Parabola fitting of MSD obtained from the optical tracking of AR in different frequencies. I AR writes “W” under RMF manipulation. J Video snapshot of AR performing a predefined track of “W”. The full name of MSD is mean square-displacement. Data in D is the mean ± S.D. Source data are provided as a Source Data file.
AMB-1 and AR were then added into the confocal dish respectively, and the motion was recorded by inverted microscope, followed by moving trajectory analysis with ImageJ software. Figure 3D illustrates the relationship between the forward velocity of AMB-1 and AR in SLF and the rotation frequency of a 7 mT magnetic field. The velocity increased proportionally with frequency up to 24 Hz, accompanied by longer displacements, as revealed by trajectory analysis. Beyond 24 Hz, however, AMB-1 and AR failed to synchronize with the rotating field, resulting in a marked decline in propulsion speed, identifying 24 Hz as their step-out frequency. This loss of synchrony is likely due to the increasing dominance of viscous resistance over magnetic torque at higher rotational frequencies47,48.
The representative tracking trajectories of AMB-1 and AR under different input frequencies of magnetic field in SLF were presented in Fig. 3E–G. It could be seen that the motion of AMB-1 and AR was directional and controllable. As showed in Fig. 3F, H, mean square-displacement (MSD) exhibited a parabolic fitting plot, further indicating the directional motion of AMB-1 and AR under magnetic manipulation. Compared with the velocity of AMB-1, the introduction of CurNPs@2TM slightly decreased the forward velocities of AR (Fig. 3D, Supplementary Fig. 10–11). Meanwhile, by modulating the RMF, AR could be precisely guided along user-defined trajectories at the microscale. As demonstrated in Fig. 3I, J and Supplementary Movie 1, AR successfully navigated a preprogrammed “W”-shaped path in real time. These findings confirm that AR exhibit highly controllable and efficient motility, a key prerequisite for their potential application in magnetically guided microrobotic therapy for pneumonia.
In vivo lung retention of AR
Before investigating the pneumonia therapeutic efficacy, the lung retention of AR was first carried out on C57 mice. The mice were randomly divided and intratracheally (i.t.) inhaled with different samples49. The mice with magnetic actuation (+M) group were placed in the center of the homemade triaxial Helmholtz coil for 30 min after 1 h of i.t. inhalation (Fig. 4A). Benefiting from the active motion in alveoli actuated by RMF, AR were supposed to penetrate into the pulmonary mucus and promote the retention effect in lung in vivo (Fig. 4B). In order to investigate how magnetic motion enhances the penetration into the lung mucus, AR were labeled with Cy5 and administered into the lungs of the mice. After magnetic treatment, the lung tissue was collected for sectioning. The results showed that under the action of RMF, AR could penetrate lung mucus and reach the lung epithelium (Supplementary Fig. 12). Based on the above results, we speculate that the triaxial Helmholtz coil induces a translational corkscrew motion of AR, which can promote the mucus penetration and subsequent drug delivery to lung epithelial cells.
A Schematic of the experiment setup. C57 mouse was i.t. inhaled with CurNPs@2TM or AR using micro-sprayer aerosolizer. The mice of AR + M group were placed in the center of the homemade triaxial Helmholtz coil for magnetic actuation for 30 min after 1 h post-inhalation. B Schematic illustration of AR-mediated mucus penetration and drug release in the lung. C Real-time FL images of C57 mice with or without magnetic field after lung administration of ICGNPs@2TM and ICGNPs@2TM-AMB-1. D Semiquantitative analysis at different time intervals based on Fig. 4C (n = 3 independent samples). E Ex vivo FL images of the major organs at 72 h after lung administration. F Semiquantitative analysis of the collected lungs based on Fig. 4E (n = 3 independent samples). G Histological section of the lung at 72 h after AR administration (Cur, green; cell nuclei, blue). In C, E, and G, the experiments were repeated independently three times with similar results, and a representative result is shown. ICG denotes indocyanine green. Data in D-F are presented as mean ± S.D. P values were analyzed by one-way ANOVA analysis followed by Tukey’s post-test (***P < 0.001, ****P < 0.0001). Source data are provided as a Source Data file.
Indocyanine green (ICG) with excitation wavelength in NIR region was used to substitute for Cur during the particle synthesis, followed by hybrid cell membrane coating and AMB-1 conjugation, here referred as ICGNPs@2TM-AMB-1. Leveraging the fluorescence from ICG, in vivo lung distribution of ICGNPs@2TM-AMB-1 was evaluated after i.t. administration at different timepoints using in vivo imaging system (IVIS). As shown in Fig. 4C, stronger FL signal was clearly observed for the mice treated with ICGNPs@2TM-AMB-1 plus magnetic field (ICGNPs@2TM-AMB-1 + M), which was retained for at least 72 h. However, the signal of the mice dosing with ICGNPs@2TM was sharply decreased in 24 h and nearly undetectable at 48 h, underscoring the role of active motion in boosting robust lung distribution and retention. The fluorescence intensity was further semi-quantified. The intensity of ICGNPs@2TM-AMB-1 + M group was the highest and was over 2-fold higher than that without magnetic treatment (Fig. 4D). The normalized fluorescence data further verified the slower clearance of ICGNPs@2TM-AMB-1 + M.
After 72 h post-administration, the mice were sacrificed and the organs were collected for ex vivo imaging. Negligible fluorescence of ICGNPs@2TM was observed in lung tissue, while ICGNPs@2TM-AMB-1 + M group came off best in lung retention (Fig. 4E), thanks to the active motion performance actuated by RMF. The semi-quantified FL intensity showed that the lung tissue retention of ICGNPs@2TM-AMB-1 + M was approximately 3-fold higher than that without magnetic actuation (Fig. 4F), suggesting the motion enhanced penetration of pulmonary mucosa and subsequent accumulation of magnetobacterial microrobot. Cur release and retention from AR were also evaluated and the lungs were collected for tissue section after i.t. inhalation for 72 h. As shown in Fig. 4G, compared with CurNPs@2TM and AR without RMF, much stronger FL signal was clearly observed for AR combined with RMF manipulation.
In vitro PsV inhibition and inflammatory cytokines neutralization
After confirming the lung retention of biomimetic AR in the presence of magnetic field, we then tested their viruses and inflammatory cytokines neutralization effects. Due to the cloaked hybrid cell membrane, AR inherited abundant ACE2 (for SARS-CoV-2) and cytokine receptors (for IL-6 and GM-CSF) from the source ACE2-293T cells and THP1 cells, enabling effective intervention of pneumonia by competing with the host cell to neutralize viruses and inflammatory cytokines (Fig. 5A, B). Firstly, the biological safety of AR was evaluated by a Cell Counting Kit-8 (CCK8) assay. AR with AMB-1 concentration of 108 cfu per mL and Cur content of 10 μg mL-1 were incubated with ACE2-293T cells and no significant cytotoxicity was observed as shown in Fig. 5C, indicating superior biosafety of AR. The results showed that after co-incubation with AR, the cell survival rate was greater than 90%. Pseudotyped SARS-CoV-2 with S proteins (pseudovirus, PsV) was then synthesized according to the previous protocol50, and incubated with ACE2-293T cells in the presence of AMB-1, CurNPs@2TM or AR. The cells co-treated with PsV and PBS or AMB-1 was infected by virus (Fig. 5D) because of the absence of ACE2 receptors. AR with hybrid cell membrane cloaking overexpressed with ACE2 was capable of competing with ACE2-293T cells to bind PsV, thus inhibiting the PsV infection efficiently, which was comparable with CurNPs@2TM. It suggested that the introduced PsV neutralization of AR via click chemistry were not affected.
A–B Schematic of AR with abundant ACE2 and cytokine receptors for A, PsV inhibition and B, inflammatory cytokines neutralization. C Cell viability of AR. ACE2-293T cells were treated with PBS, AMB-1, CurNPs@2TM and AR (the concentration of AMB-1 was 108 cfu mL−1, and Cur was 10 μg mL-1) (n = 3 independent samples). D In vitro luminescence images and semiquantitative analysis of ACE2-293T cells after PsV infection (n = 3 independent samples). E–F In vitro E, IL-6 and F, GM-CSF neutralization by AR (n = 3 independent samples). G Schematic representation of the experiment setup. PsV infected mouse model was constructed by lung administration of PsV. 0.5 h after PsV challenge, the mice were inhaled with PBS, AMB-1, CurNPs@2TM and AR. The mice from AMB-1 + M group and AR + M group were placed in magnetic field for 30 min at 1 h after lung administration of AMB-1 or AR. H Bioluminescence analysis of PsV infection inhibition of mice after different treatments. The experiment was repeated independently three times with similar results, and a representative result is shown. I Semiquantitative analysis of bioluminescence intensity of PsV infected mice at different time (0 h, 48 h and 72 h) (n = 3 independent samples). J Representative H&E images of PsV infected lung sections 48 h after different treatments. The experiment was repeated independently three times with similar results, and a representative result is shown. K Histogram of lung injury scores after different treatments (n = 3 independent samples). L–M Inflammatory factors including L, IL-6 and M, GM-CSF in the BALF after treatments (n = 3 independent samples). PsV, FL, IL-6, GM-CSF, and BALF refer to pseudovirus, fluorescence, interleukin-6, granulocyte–macrophage colony-stimulating factor, and bronchoalveolar lavage fluid, respectively. Data in C–F, I, and K–M are presented as mean ± S.D. P values were analyzed by one-way ANOVA analysis followed by Tukey’s post-test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Source data are provided as a Source Data file.
The capability of AR to neutralize inflammatory cytokines was further investigated. AR was first incubated with 100 μL of PBS containing known initial concentrations of IL-6 (2 μg mL−1) and GM-CSF (0.5 μg mL−1) for 30 min. After removal of AR by centrifugation, the supernatant was analyzed by ELISA to determine residual cytokine concentration23. Benefiting from the abundant cytokine receptors expressed on CurNPs@2TM and AR, we demonstrated that CurNPs@2TM and AR could neutralize IL-6 and GM-CSF efficiently, suggesting potential of AR for attenuating cytokine storm (Fig. 5E, F). While the current system targets IL-6 and GM-CSF, the broad expression of cytokine receptors on the AR surface suggests its applicability for neutralizing a wider spectrum of pro-inflammatory cytokines.
In vivo PsV inhibition and inflammatory cytokines neutralization of AR
Encouraged by effective PsV inhibition and inflammatory cytokines neutralization of AR in vitro, in vivo evaluation was further conducted. HACE2-mice with overexpression of ACE2 were used in this study. HACE2-mice pre-inhaled with PsV using micro-sprayer aerosolizer were divided into six groups randomly: PBS group, AMB-1 group, AMB-1 + M group, CurNPs@2TM group, AR group and AR + M group. After 0.5 h post-administration of PsV, the mice were then inhaled with AMB-1, CurNPs@2TM or AR. The mice from AMB-1 + M group and AR + M group were placed in the center of the homemade triaxial Helmholtz coil for magnetic actuation for 30 min after 1 h of i.t. inhalation of AMB-1 or AR (Fig. 5G). Real-time in vivo bioluminescence imaging was performed to monitor PsV infection, with semi-quantitative analysis of fluorescence intensity. The higher fluorescence intensity indicates the worse PsV infection. As illustrated in Fig. 5H, I, the results showed the weakest bioluminescence signal in AR + M group among all the groups, reflecting the potent PsV inhibition. Compared with AR group, the PsV inhibition of mice treated with AR + M exhibited a pronounced decrease in total fluorescent signal, and the decrease in total fluorescence was 10 times lower than that of the AR group.
After treatment, the lung was collected and the histopathological analysis of lung sections was further conducted. As shown in Fig. 5J, PsV infection induced pneumonia as revealed by morphological damage. Notably, we found that compared to the AR group, the AR + M group significantly alleviated pneumonia. Moreover, we evaluated lung tissue slices and found that treatment with AR moderately inhibited the PsV-induced pulmonary injury, while AR combined with magnetic actuation (AR + M) achieved markedly improved therapeutic efficacy, as reflected by the lowest lung injury score (Fig. 5K). Bronchoalveolar lavage fluid (BALF) was collected for cytokine profiling, showing elevated IL-6 and GM-CSF levels following PsV exposure and AR + M obviously decreased the IL-6 and GM-CSF levels in BALF (Fig. 5L, M), demonstrating potential of AR + M for broad-spectrum cytokine neutralization. We speculate that this is because the magnetotactic movement of AR in the alveoli enhances the contact between AR and inflammatory factors, thereby improving the neutralization effect of inflammatory factors. In addition, the active motion of AR in the magnetic field enhances their penetration into the pulmonary mucus layer, thus reaching epithelial cells to release Cur and promoting drug retention in the lungs. Under the action of Cur, the release of inflammatory factors in alveoli can be reduced.
AR suppress acute pneumonia
After confirming the cytokine neutralization of AR + M in vivo, we further assessed the ability of AR + M to treat acute pneumonia. Acute pneumonia refers to an acute lung infection caused by pathogens such as bacteria and viruses, often accompanied by symptoms such as fever, cough, and difficulty breathing, which require timely treatment. To induce acute pneumonia, C57 mice were i.t. inhaled with Lipopolysaccharides (LPS) (10 mg mL−1)33. The mice pre-treated with LPS were randomly assigned to six groups: PBS group, AMB-1 group, AMB-1 + M group, CurNPs@2TM group, AR group and AR + M group. Subsequently, the mice were i.t. inhaled with AMB-1, CurNPs@2TM or AR 3 h after LPS challenge. The mice from AMB-1 + M group and AR + M group were then positioned at the center of the Helmholtz coil for magnetic actuation for 30 min after 1 h of AMB-1 or AR administration (Fig. 6A). Cur has been proven to have reactive oxygen species (ROS) scavenging and anti-inflammatory effects, and is widely used in the treatment of various inflammatory diseases39,51,52. Therefore, ROS production of acute pneumonia mice was investigated. A ROS sensitive probe L-012 (25 mg kg−1) was first injected into the mice intravenously to evaluate the oxidative stress surrounding the injured lung 24 h after LPS inhalation. The inflammation was recorded by IVIS. As shown in Fig. 6B, the lung tissue of PBS group showed strong bioluminescence signals, while the lung of AR + M group only had negligible bioluminescence signals and were much weaker than the bioluminescence signals of the AR group. The reason for this result is that under the action of magnetic field, AR increases the retention of Cur in lung tissue, further promoting ROS clearance. The semi-quantitative analysis of lung tissue bioluminescence intensity showed that AR + M group had a decrease of about 12 times compared to the PBS group and about 6 times compared to the AR group, further indicating that AR + M treatment significantly alleviated the LPS induced acute pneumonia (Fig. 6C).
A Schematic of the experiment setup. Mouse model was constructed by lung challenge of LPS (10 mg kg−1). 3 h following LPS administration, mice were inhaled with PBS, AMB-1, CurNPs@2TM and AR. Mice from AMB-1 + M group and AR + M group were treated in magnetic field for 30 min at 1 h after AMB-1 or AR administration. B FL imaging of the lungs of mouse after injecting ROS probe (L-012) at 24 h after LPS challenge. The experiment was repeated independently three times with similar results, and a representative result is shown. C Semiquantitative of FL intensity of the lungs of ALI mouse after injecting L-012 (n = 3 independent samples). D Representative H&E images of lung sections after different treatments. The experiment was repeated independently three times with similar results, and a representative result is shown. E Histogram of lung injury scores (n = 3 independent samples). F W/D weight ratio of lung tissue (n = 3 independent samples). G–H ELISA analysis of inflammatory factors including G, IL-6 and H, GM-CSF in BALF after treatments (n = 3 independent samples). I M2 type macrophage in lung slice after different treatments (Red: Cy3-F4/80, Green: Cy5-CD206, Blue: DAPI). The experiment was repeated independently three times with similar results, and a representative result is shown. J Quantitative analysis of macrophages (F4/80+ CD11b+ cells) in lung after treatment (n = 3 independent samples). K Flow cytometry quantification of M2 macrophages (F4/80+ CD11b+ CD206+) (n = 3 independent samples). L Flow cytometry quantification of M1 macrophages (F4/80+ CD11b+ CD11c+ cells) (n = 3 independent samples). LPS, IL-6, GM-CSF, and BALF refer to lipopolysaccharide, interleukin-6, granulocyte-macrophage colony stimulating factor, and bronchoalveolar lavage fluid, respectively. Data in C, E–H, and J–L are presented as mean ± S.D. P values were analyzed by one-way ANOVA analysis followed by Tukey’s post-test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Source data are provided as a Source Data file.
All mice were euthanized at 24 h after LPS challenge for histological analysis to investigate the therapeutic effect. LPS-challenged mice exhibited marked alveolar wall thickening, loss of alveolar spaces, vascular dilation and congestion, and extensive inflammatory cell infiltration, confirming severe lung injury in the PBS group (Fig. 6D). Notably, AR + M significantly suppressed the lung injury, further demonstrating that active AR under magnetic field was highly effective in pneumonia treatment. The histological score was also analyzed based on Mikawa method33,53. As shown in Fig. 6E, PBS group, AMB-1 group and AMB-1 + M group exhibited high histological scores, reflecting severe inflammation. However, the lung tissue slices of AR + M group had the smallest injury score, demonstrating significant therapeutic efficacy on pneumonia mice. Besides, the severity of pulmonary edema in mice with acute pneumonia was evaluated by measuring the lung wet/dry (W/D) weight ratio. The lung W/D weight ratio reflects the water content of the lungs, indirectly reflecting inflammation, edema, or other pathological changes of the lungs. Due to increased exudate caused by acute pneumonia, the lung W/D weight ratio will increase, which is an auxiliary parameter for evaluating the severity of pneumonia and treatment effectiveness. The decrease of the lung W/D weight ratio indicates that pneumonia has been relieved. As expected, AR + M performed the greatest degree of remission (Fig. 6F).
Furthermore, lung BALF was further collected for the relevant inflammatory cytokine quantification. IL-6 and GM-CSF levels in PBS group were significantly increased, which showed severe inflammatory status of mice model. IL-6 and GM-CSF levels in AMB-1 group and AMB-1 + M group were comparable to those in PBS group, indicating that AMB-1 had no inflammatory therapeutic effect. Remarkably, AR + M effectively decreased the levels of IL-6 and GM-CSF in BALF, with a clearance rate of inflammatory factors exceeding 85%, suggesting the encouraging therapeutic efficacy of AR + M on the treatment of pneumonia (Fig. 6G, H). The above results all indicated effective pneumonia recover, which was attributed to the active movement of AR under the action of magnetic field, achieving rapid neutralization of inflammatory factors in the pneumonia microenvironment, and the effective retention and release of Cur by AR under the action of magnetic field, inhibiting the production of inflammation factors.
AMs polarization and biosafety evaluation
Macrophage reprogramming and polarization in the lung play a pivotal role in the onset, progression, and severity of pneumonia. Modulating pulmonary macrophage activation and differentiation thus represents a potential therapeutic strategy for the disease. Reducing the infiltration of macrophages or polarizing macrophages into M2 phenotype can effectively alleviate pulmonary inflammation1,7,16. To investigate the regulatory role of AR + M in the anti-inflammatory process of acute pneumonia, the lung tissues were collected and sliced after treatment. Immunostaining targeting F4/80 (red) and CD11c (green) or F4/80 (red) and CD206 (green), the markers of pro-inflammatory M1 macrophages or anti-inflammatory/reparative M2 macrophages respectively was used to employed to assess the immunomodulatory effect of AR + M on macrophage polarization in vivo. As shown in Fig. 6I and Supplementary Fig. 13, a large amount of M1 type macrophage (F4/80+, CD11c+ cells) and a small amount of M2 type macrophage (F4/80+, CD206+ cells) were observed for acute pneumonia mice treated with PBS, reflecting the pulmonary inflammatory microenvironment. However, a marked increase in M2 macrophages was observed, accompanied by a reduction in M1 macrophages in the AR + M group, indicating effective modulation of pulmonary inflammation. The reason for this result is that AR increases lung retention under the action of a magnetic field, thereby increasing the amount of Cur in the lungs for alleviation of the pneumonia microenvironment.
Single-cell suspensions of lung tissues were analyzed by flow cytometry to quantify M1 and M2 macrophage populations. As shown in Fig. 6J and Supplementary Fig. 14–15, the number of macrophages (F4/80+, CD11b+) was reduced in the AR + M group compared to PBS, indicating a mitigated inflammatory microenvironment. As shown in Fig. 6K, L and Supplementary Fig. 16–19, AR + M treatment markedly increased the proportion of M2 macrophages (F4/80+, CD11b+, CD206+) while reducing the proportion of M1 macrophages (F4/80+, CD11b+, CD11c+). The above results further suggest that under the action of magnetic field, AR increases the retention of Cur in lung tissue, enhances immunomodulatory effect of Cur, reduces macrophage infiltration and M1 macrophages, and polarizes macrophages into M2 type, thereby regulating the pneumonia microenvironment and promoting pneumonia treatment.
Biosafety of AR + M was also studied. To evaluate the potential hepatorenal toxicity of AR + M, serum liver (ALT, AST) and kidney (UREA, CREA-S) function markers were measured at the end of treatment. (Supplementary Fig. 20). No significant differences were observed among groups, indicating favorable biocompatibility of AR and magnetic actuation. Additionally, major organs were harvested and subjected to H&E staining for histopathological assessment (Supplementary Fig. 21). H&E staining of heart, liver, spleen, lung, and kidney tissues revealed no apparent histopathological abnormalities across all groups, further supporting the excellent biocompatibility of AR and magnetic actuation. To further ensure the safety of AR, the lungs of the mice were collected on day 1, 2, 3 and 4 after lung administration of AR with RMF treatment. AMB-1 colonies of the lungs tissue were tested and we found that AMB-1 in the lung was metabolized within 3 days (Supplementary Fig. 22). Other main organs including heart, liver, spleen, and kidneys were also detected and no AMB-1 colonies were observed, indicating that the live magnetotactic bacteria could not migrate from the lungs to other organs (Supplementary Fig. 23). H&E was also performed on the organs of the mice 3 days after pulmonary administration of AR with RMF. No damage of tissues was observed, further demonstrating the acute safety of pulmonary administration of AR (Supplementary Fig. 24A). Serum hepatic (ALT, AST) and renal (UREA, CREA-S) function markers were also assessed 3 days post-treatment (Supplementary Fig. 24B, C) and no significant difference were observed, further indicating the biosafety of AR with magnetic actuation.
Discussions
In this study, “biomimetic” refers to the integration of natural biological components into the microrobot design to enhance the functionality, biocompatibility, and therapeutic efficacy. We develop natural magnetotactic bacterium based biomimetic microrobots for active and efficient pneumonia therapy. By using metabolic glycan labelling strategy, azide functional groups are successfully introduced into the surface of cell membrane and the obtained azide modified hybrid membrane is then utilized to coat Cur-loaded nanoparticles; Meanwhile, DBCO is also introduced on the surface of magnetotactic bacteria, and a biomimetic magnetotactic bacterium based microrobot modified with hybrid cell membrane cloaking nanoparticles is constructed by click chemistry. Based on specific properties of the modified hybrid cell membrane, the biomimetic robot can effectively eliminate lung inflammatory factors and viruses along its path of progress and block the further activation of inflammation. The active motion of the biomimetic robot in the confined space of lungs can further enhance the deep tissue penetration of Cur, and then play a better role in treatment and regulation of the pneumonia microenvironment. In addition, we further evaluate the biosafety of the magnetic bacteria. In vitro cell experiments and in vivo animal experiments have confirmed its good biocompatibility.
Compared with traditional cell membrane cloaked nanoparticles, AR combines cell membrane coating nanotechnology with self-propelled microrobots, utilizing the active motion characteristics of robots to improve the effective neutralization of viruses and inflammatory factors in the confined lung environment. The magnetic actuated microrobots also promote deep tissue penetration of the loaded anti-inflammatory Cur, thereby achieving enhanced and active pneumonia treatment and pneumonia microenvironment regulation. Meanwhile, the biomimetic properties of the cell membrane coating endow the robot with immune escape function, effectively avoiding active clearance by alveolar macrophages, thereby prolonging the tissue retention of the drugs. It can also ensure the biological safety due to its negligible toxicity, as evidenced by our in vivo observations of controlled immune responses. By leveraging these biomimetic strategies, our microrobot system achieves enhanced targeting, prolonged retention, and improved therapeutic outcomes for pneumonia treatment.
This study delves into the mechanism of active drug delivery regulation of pneumonia by magnetobacterial microrobots, which not only broadens the application of biomimetic microrobots in biomedical fields, but also provides a new theoretical basis and research foundation for active and precise treatment of pneumonia. Under an applied rotating magnetic field, the microrobots demonstrate precise and programmable motion in vitro, exhibiting translational potential for targeted delivery to lesions (e.g., inflammatory sites, tumors) in vivo when integrated with real-time imaging-guided navigation systems, such as ultrasound or X-ray fluoroscopy. And it is expected to further expand the practical biomedical application scope of microrobots in a broader perspective. Although magnetic fields can guide the movement direction of magnetic bacteria, the strength and distribution of the magnetic field may be affected by various factors, such as the density and the thickness of the lung tissue, which can affect the control accuracy of magnetic bacteria. With the continuous advancement and improvement of technology, we believe that the prospects of magnetic bacterial robots in lung applications will be even broader.
Methods
Cell lines and animals
Human myeloid mononuclear THP-1 cells (SCSP-567) were obtained from the Cell bank of Chinese Academy of Sciences (Shanghai, China). Genetically transfected ACE2-293T cells (41107ES03) were purchased from Yeasen Biotechnology (Shanghai, China). Human ACE2 transgenic mice (hACE2-mice) and C57 mice were used in the study. The mice were permitted to consume food and water at their discretion, and they were maintained in an ambient temperature range of 21–26 °C, with a humidity level of 50-60%, and a 12-h light/dark cycle. All animal procedures were performed in accordance with the Guidelines for the Institutional Animal Care and Use Committee (IACUC) and approved by the Animal Ethics Committee of Southern Medical University (Permit Number: SMUL-202311053).
Materials
Magnetotactic bacteria AMB-1 (ATCC 700264) was obtained from Biobw (Beijing, China). This wild-type, Gram-negative, facultatively anaerobic, magnetotactic bacterium possesses a fully sequenced genome and biomineralizes magnetosomes. All experiments were performed using the parental strain without further genetic modification. Cur and LPS was bought from Sigma-Aldrich (USA). DBCO-PEG4-NHS ester was bought from Guangzhou Tanshtech Co., Ltd (Guangzhou, China). Ac4GalNAz was purchased from Macklin (Shanghai, China). HSA was obtained from Bomeibio (Hefei, China).
Preparation of 2TM
THP-1 cells were cultivated in RPMI 1640 media, and ACE2-293T cells were cultured in high glucose DMEM media. All media contain 10% fetal bovine serum with 1% penicillin and streptomycin. Azido sugar Ac4GalNAz (50 μM) was added into THP-1 cells and ACE2-293T cells respectively. After incubating for 48 h, activated azide (N3)-labeling THP-1 cells (THP1-N3) and ACE2-293T cells (ACE2-293T-N3) were then obtained.
Cell membrane was obtained according to the previous report54,55,56. Briefly, THP1-N3 cells or ACE2-293T-N3 cells were lysed, followed by mechanical membrane disruption (Huxi JY92-IIDN, China), and 1 h ice-cold incubation. The resulting solutions were then conducted with differential centrifugation (Beckman Optima XPN-100 ultracentrifuge, USA), and the THP1-N3 cell membrane (TM) and ACE2-293T-N3 cell membrane (2 M) were obtained and stored at −80 °C.
By adding 500 μL of ultrapure water to 2 M and TM respectively, the isolated cell membranes were re-suspended and mixed at a 1:1 protein mass ratio (2 M:TM). The hybrid membrane (2TM) was then prepared by sonication for 20 s followed by repeated extrusion through a 220 nm polycarbonate membrane.
Fabrication of CurNPs@2TM and ICGNPs@2TM
CurNPs were prepared by adding 200 μL of curcumin solution (2.5 mg mL−1 in dimethyl sulfoxide) dropwise into 4 mL of HSA (2.5 mg mL−1 in pure water) under sonication57. CurNPs were then obtained by ultrafiltration with pure water for three times. And the mixture of 2TM and CurNPs were physically squeezed repeatedly through 220 nm polycarbonate membrane for the formation of CurNPs@2TM.
ICGNPs were prepared by slowly adding 200 μL of ICG solution (2.5 mg mL−1 in dimethyl sulfoxide) into 4 mL of HSA solution (2.5 mg mL−1 in water) under continuous sonication. ICGNPs were then obtained by ultrafiltration with pure water for three times. The 2TM and ICGNPs mixture was repeatedly extruded through a 220 nm polycarbonate membrane to generate ICGNPs@2TM.
Preparation of AR and ICGNPs@2TM-AMB-1
AMB-1 (107 CFU mL−1) were cultured in enriched magnetic spirillum growth medium (EMSGM) at 30 °C in the dark58. AMB-1 were washed three times with pure water to remove residual medium and then resuspended in pure water. Then, 1 × 108 of AMB-1 were incubated with 0.1 mg mL−1 of DBCO-PEG4-NHS for 1 h at room temperature to acquire DBCO-labeled AMB-1 (AMB-1-DBCO). To remove unreacted DBCO-PEG4-NHS, AMB-1-DBCO were washed three times with pure water.
AMB-1-DBCO were incubated with azido-functionalized CurNPs@2TM at room temperature for 1 h. After 10 min centrifugation at 5,000 x g and washing with PBS for three times, the resulting CurNPs@2TM-AMB-1 (AR) were collected.
AMB-1-DBCO were incubated with azido-functionalized ICGNPs@2TM at room temperature for 1 h. After 10 min centrifugation at 5000 x g and washing with PBS for three times, the resulting ICGNPs@2TM-AMB-1 were collected.
Characterization of CurNPs@2TM and AR
The morphology of CurNPs, CurNPs@2TM, AMB-1, and AR was characterized using scanning electron microscopy (SEM, Quattro S, Thermo, USA), transmission electron microscopy (TEM, JEOL-1400, Japan), and fluorescence microscopy (Eclipse Ti2, Nikon, Japan). The absorption spectrum was recorded using a UV-vis spectrometer (Shimadzu UV-2600, Japan) and the fluorescence spectrum was obtained using a fluorescence spectrophotometer (Shimadzu RF-6000, Japan). The hydrodynamic diameters of CurNPs and CurNPs@2TM were measured by dynamic light scattering (DLS, Malvern Zetasizer Nano ZSE, UK). The zeta potential of CurNPs, 2TM, CurNPs@2TM, AMB-1 and AR were also measured by DLS. Room-temperature magnetic properties of AMB-1 and AR were measured using a physical property measurement system (PPMS-9; Quantum Design, USA).
THP-1 and ACE2-293T cells pretreated with or without Ac4GalNAz (50 μM) were incubated with fluorescent DBCO-Cy5, and the fluorescence intensity was obtained by fluorescence microscope imaging and flow cytometric analysis (BD LSRFortessa X-20, USA).
SDS-PAGE was performed to analyze the membrane proteins of CurNPs@2TM. Membrane proteins were extracted from 2 M, TM, 2TM, and CurNPs@2TM using RIPA lysis buffer, and total protein concentration was quantified by BCA assay (Beyotime, China). Samples (100 μg protein per lane) were denatured at 100 °C for 10 min in SDS loading buffer, then separated on 7.5% SDS-PAGE gels using the Mini-PROTEAN Tetra System (Bio-Rad, USA). Proteins were transferred onto PVDF membranes at 240 mA for 2 h. Membranes were incubated overnight at 4 °C with primary antibodies against ACE2 (Santa Cruz Biotechnology, sc-390851), IL6ST (ABclonal, A18036), CD116 (ABclonal, A23001), and ATP1A1 (Proteintech, 14418-1-AP), followed by 1 h incubation at 37 °C with HRP-conjugated secondary antibodies (anti-rabbit IgG-Proteintech, SA00001-2; anti-mouse IgG-Proteintech, SA00001-1). Protein bands were visualized using the ChemiDoc MP imaging system (Bio-Rad).
Propidium iodide (PI) dye was used to mark dead AR 1 day after AMB-1 loaded with CurNPs@2TM. And we diluted AR and coated it on a EMSGM solid-state culture plate to detect the survival rate of AR. AR density was quantified by measuring optical density at 600 nm (OD600) and referencing a standard curve.
Motion evaluation of AR under magnetic field
The controllable motion of AMB-1 and AR was actuated by rotating magnetic field generated by a homemade three-dimensional Helmholtz coil system. AMB-1 and AR with appropriate concentration were dispersed in simulated lung fluid (SLF) in a confocal dish. Samples were positioned at the center of a Helmholtz coil to acquire a uniform magnetic field and imaged using a Nikon Ti2-A microscope. Time-lapse images were recorded with ΔT of = 200 ms. These image sequences were analyzed by ImageJ software with manual tracking plugin. The motion of AMB-1 and AR was manipulated by changing the frequency and the phase of the rotating magnetic field.
In Vivo detection of AR in lung
In order to observe how magnetic motion enhances the penetration into the lung mucus, AR were labeled with Cy5. In short, Cy5-maleimide was conjugated to activated sulfhydryl groups on the surface of AMB-1 bacteria via Michael addition. Next, DBCO was labeled on the surface of Cy5 labeled AMB-1, and the resulting bacteria were connected with N3 labeled CurNPs@2TM, ultimately forming Cy5 labeled AR. C57 mice were assigned to two groups randomly (AR group and AR + M group). The mice were intratracheally (i.t.) inhaled with Cy5 labeled AR. The mice from AR + M group were positioned in the center of the homemade triaxial Helmholtz coil for magnetic actuation for 30 min after 1 h of i.t. inhalation of AR. The mice were sacrificed after magnetic actuation. Lungs were harvested and fixed in 4% paraformaldehyde for frozen sectioning. Sections were stained with DAPI and imaged using DAPI and Cy5 filters.
C57 mice were assigned to three groups randomly (ICGNPs@2TM group, ICGNPs@2TM-AMB-1 group and ICGNPs@2TM-AMB-1 + M group). The mice were intratracheally (i.t.) inhaled with ICGNPs@2TM or ICGNPs@2TM-AMB-1 using micro-sprayer aerosolizer (YUYANBIO, Shanghai, China). The mice from ICGNPs@2TM-AMB-1 + M group were placed in the center of the homemade triaxial Helmholtz coil for magnetic actuation for 30 min after 1 h of inhalation. In vivo imaging system (IVIS) was used to record the retention of the samples in lung at different times after inhalation (3 h, 12 h, 24 h, 48 h, 72 h) (Ex: 710 nm, Em: 770 nm). The mice were sacrificed after 72 h of i.t. inhalation. The major organs were collected and sectioned for FL imaging to detect the retention of ICGNPs@2TM or ICGNPs@2TM-AMB-1.
C57 mice were assigned to three groups randomly (CurNPs@2TM group, AR group and AR + M group). The mice were intratracheally (i.t.) inhaled with CurNPs@2TM or AR. The mice from AR + M group were placed in the center of the homemade triaxial Helmholtz coil for magnetic actuation for 30 min after 1 h of i.t. inhalation of AR. The mice were sacrificed after 72 h of i.t. inhalation. Lungs were collected and fixed in 4% paraformaldehyde for frozen sectioning. Sections were stained with DAPI and imaged using DAPI and FITC filter sets.
Prepared of pseudovirus (PsV)
PsV were developed following the procedures below. 293T cells were co-transfected with pCDH-Luciferase, psPAX2, and pCAGGS-SARS-2-S-flag plasmids encoding viral glycoproteins. Pseudovirus (PsV) was harvested from the supernatant 48 h post-transfection and concentrated by ultracentrifugation at 100,000 × g through a 20% sucrose cushion for 2.5 h at 4 °C using an Optima XPN-100 ultracentrifuge (Beckman Coulter, USA). The resulting PsV pellet was resuspended in PBS and stored at −80 °C.
In vitro Neutralization of PsV and Inflammatory Cytokines
ACE2-293T cells were seeded in 24-well plates at 1 × 105 cells per well and cultured for 24 h. Prior to infection, 5 μL of PsV was mixed with 500 μL of culture medium containing PBS, AMB-1, CurNPs@2TM and AR (the concentration of AMB-1 was 107 cfu per mL, and Cur was 1 μg mL−1) respectively. Then 500 μL of the mixture was added into ACE2-293T cells, and the culture media were replaced after 12 h. After an additional 48 h of incubation, the Luciferase Assay System (AAT Bioquest, USA) was employed to analyze the luciferase activity.
To determine the cytokine binding effect of AR, 100 μL of PBS containing AMB-1, CurNPs@2TM or AR were incubated with 100 μL of PBS containing IL-6 (2,000 pg mL−1) or GM-CSF (500 pg mL−1) for 30 min. Samples were then centrifuged at 15,000 × g for 15 min to remove nanoparticles, and cytokine levels in the supernatant were quantified using IL-6 or GM-CSF ELISA kits (BioLegend, USA).
In vivo neutralization of PsV and inflammatory cytokines
HACE2-mice pre-inhaled with PsV were randomly divided into six groups (PBS, AMB-1, AMB-1 + M, CurNPs@2TM, AR, AR + M). The mice were then i.t. inhaled with AMB-1, CurNPs@2TM or AR using micro-sprayer aerosolizer after 0.5 h of PsV inhalation. The mice from AMB-1 + M group and AR + M group were placed in the center of the homemade triaxial Helmholtz coil for magnetic actuation for 30 min after 1 h of i.t. inhalation of AMB-1 or AR.
After inhaling PsV at different times (0 h, 48 h, and 72 h), d-fluorescein (150 mg kg−1) was intravenously injected for the quantification of the PsV infection in lung tissue using IVIS system.
The mice were sacrificed 72 h after PsV inhalation, and lungs were collected, fixed in 4% paraformaldehyde, and processed for frozen sectioning followed by H&E staining. Lung injury was scored using the Mikawa method33,53 based on four parameters graded from 0 to 4: alveolar congestion, pulmonary hemorrhage, neutrophil infiltration, and septal thickening. The sum of these scores represented the overall lung injury.
72 h after PsV inhalation, mice were euthanized and positioned on an endotracheal intubation platform. The lungs were lavaged three times with 0.8 mL of ice-cold PBS via a tracheal catheter. Bronchoalveolar lavage fluid (BALF) was collected and centrifuged at 15,000 × g for 10 min at 4 °C, and the supernatant was harvested for subsequent analysis. The BALF levels of IL-6 and GM-CSF were measured by corresponding ELISA kits.
In vivo inhibition of acute pneumonia
C57 mice pre-inhaled with LPS (10 mg mL) were randomly divided into six groups (PBS, AMB-1, AMB-1 + M, CurNPs@2TM, AR, AR + M). The mice were then i.t. inhaled with AMB-1, CurNPs@2TM or AR using micro-sprayer aerosolizer after 3 h of LPS inhalation. The mice from AMB-1 + M group and AR + M group were placed in the center of the homemade triaxial Helmholtz coil for magnetic actuation for 30 min after 1 h of AMB-1 or AR inhalation.
To assess oxidative stress in LPS-induced acute pneumonia, a reactive oxygen species–sensitive probe (L-012; 25 mg kg−1, Wako Chemicals, Japan) was administered via intravenous injection 24 h after LPS exposure. Fluorescence (FL) signals were monitored using an IVIS imaging system over a 30 min period.
The mice were sacrificed after 24 h of LPS inhalation and the lungs of each group were collected and fixed in 4% PFA and then frozen sectioned for H&E staining. Lung injury scores were also evaluated by Mikawa method33,53.
The mice were sacrificed after 24 h of LPS inhalation and the lungs of each group were collected. Harvested lung tissues were weighed immediately to obtain wet weight, then dehydrated at 65 °C for 72 h to determine dry weight. The lung wet-to-dry (W/D) weight ratio was subsequently calculated.
24 h after LPS inhalation, mice were euthanized and positioned on an endotracheal intubation platform. The lungs were lavaged three times with 0.8 mL of ice-cold PBS via a tracheal catheter. Bronchoalveolar lavage fluid (BALF) was collected and centrifuged at 15,000 × g for 10 min at 4 °C, and the supernatant was harvested for downstream analysis. The BALF levels of IL-6 and GM-CSF were measured by corresponding ELISA kits.
Analysis of M1 Type and M2 Type alveolar macrophages
Mice with acute pneumonia were sacrificed after 24 h of LPS inhalation and the lungs of each group were excised and fixed with 4% PFA. The tissues were embedded in paraffin, followed by cutting into slices with 4 μm thickness. Alveolar M1 and M2 macrophages were identified in tissue sections by co-staining with Cy3-conjugated anti-F4/80 and Cy5-conjugated anti-CD11c antibodies (for M1) or Cy3-conjugated anti-F4/80 and Cy5-conjugated anti-CD206 antibodies (for M2). Stained sections were imaged using fluorescence microscopy.
The collected lung tissues were homogenized through a nylon mesh to obtain single-cell suspensions, which were stained with PerCP-Cy5.5–anti-F4/80 (Biolegend, 123128), BV605–anti-CD11b (BD, 563015), PE–anti-CD11c (BD, 557401), and APC–anti-CD206 (Thermo, 17-2061-80) antibodies. After washing, cells were resuspended in PBS for flow cytometry analysis. M1 macrophages were defined as F4/80+CD11b+CD11c+ cells, while M2 macrophages were identified as F4/80+CD11b+CD206+ cells.
In Vivo and in vitro biosafety detection
The biosafety of AR on ACE2-293T cells was evaluated by CCK8 assay (GLPBIO, USA). ACE2-293T cells were seeded in 96-well plates at a density of 8 × 103 cells per well and cultured for 24 h. The medium was then replaced with fresh medium containing AMB-1 (108 CFU mL−1), CurNPs@2TM, or AR (Cur concentration, 10 μg mL−1). After 48 h of incubation, the supernatant was removed, and 100 μL of fresh medium containing 10% CCK-8 solution was added. Following incubation for 0.5–2 h, absorbance was measured using a multifunctional microplate reader (Spark, TECAN, Switzerland).
For in vivo toxicity evaluation, serum was collected after treatment and analyzed for liver (ALT/AST) and kidney (BUN/CREA) function using corresponding activity assay kits. Major organs including the heart, liver, spleen, lungs, and kidneys were harvested, fixed in 4% paraformaldehyde, sectioned, and subjected to H&E staining for histological analysis under a microscope.
To further ensure the safety, C57 mice were i.t. inhaled with AR (the concentration of AMB-1 was 108 cfu mL−1, and Cur was 10 μg mL−1). And then, the mice were placed in the center of the homemade triaxial Helmholtz coil for magnetic actuation for 30 min after 1 h of AR inhalation. The major organs of the mice were extracted, weighed, and homogenized in EMSGM medium on day 1, 2, 3 and 4 after lung administration of AR with RMF. These samples were diluted (10 times) and coated on EMSGM solid-state culture plate. After incubation, the colonies of the tissues in each group were quantified.
Statistical analysis
All statistical analyses were analyzed by Microsoft Excel 2019, Graphpad Prism 8.0, origin 85, and Image J (1.52a). Flow cytometry was analyzed using Flowjo_V10 software. Statistical analysis was conducted using one-way ANOVA analysis followed by Tukey’s post-test, and the date represent mean ± SD. The asterisk denoted statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The source data generated in this study are provided in the Supplementary Information/Source Data file. The full image dataset is available from the corresponding author upon request. Source data are provided with this paper. Source data are available for Figs. 2–6 and Supplementary Figs. 3–4, 6–11, 20, and 24 in the associated source data file on Figshare: https://doi.org/10.6084/m9.figshare.28944014.
References
-
Muhammad, W., Zhai, Z., Wang, S. & Gao, C. Inflammation-modulating nanoparticles for pneumonia therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 14, e1763 (2022).
-
Li, J. et al. Supramolecular erythrocytes-hitchhiking drug delivery system for specific therapy of acute pneumonia. J. Controlled Release 350, 777–786 (2022).
-
Ren, H.-M. et al. Inhalable responsive polysaccharide-based antibiotic delivery nanoparticles to overcome mucus barrier for lung infection treatment. Nano Today 44, 101489 (2022).
-
Cilloniz, C., Dela Cruz, C., Curioso, W. H. & Vidal, C. H. World Pneumonia Day 2023: the rising global threat of pneumonia and what we must do about it. Eur. Respiratory J. 62, 2301672 (2023).
-
Song, C. et al. NETs promote ALI/ARDS inflammation by regulating alveolar macrophage polarization. Exp. cell Res. 382, 111486 (2019).
-
Ruaro, B. et al. The History and Mystery of Alveolar Epithelial Type II Cells: Focus on Their Physiologic and Pathologic Role in Lung. Int. J. Mol. Sci. 22, 2566 (2021).
-
Wang, Z., Li, S. & Huang, B. Alveolar macrophages: Achilles’ heel of SARS-CoV-2 infection. Signal Transduct. Target. Ther. 7, 242 (2022).
-
Dukhinova, M., Kokinos, E., Kuchur, P., Komissarov, A. & Shtro, A. Macrophage-derived cytokines in pneumonia: Linking cellular immunology and genetics. Cytokine growth factor Rev. 59, 46–61 (2021).
-
Meng, Q. F. et al. Inhalation delivery of dexamethasone with iSEND nanoparticles attenuates the COVID-19 cytokine storm in mice and nonhuman primates. Sci. Adv. 9, eadg3277 (2023).
-
Zhou, A. et al. Bioengineered Neutrophil Extinguisher Targets Cascade Immune Pathways of Macrophages for Alleviating Cytokine Storm in Pneumonia. ACS nano 17, 16461–16477 (2023).
-
Abdollahi, E. et al. Immunomodulatory Therapeutic Effects of Curcumin on M1/M2 Macrophage Polarization in Inflammatory Diseases. Curr. Mol. Pharmacol. 16, 2–14 (2023).
-
Sun, H. et al. Modulation of Macrophages Using Nanoformulations with Curcumin to Treat Inflammatory Diseases: A Concise Review. Pharmaceutics 14, 2239 (2022).
-
Suresh, M. V., Francis, S., Aktay, S., Kralovich, G. & Raghavendran, K. Therapeutic potential of curcumin in ARDS and COVID-19. Clin. Exp. Pharmacol. Physiol. 50, 267–276 (2023).
-
Chopra, H. et al. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 26, 4998 (2021).
-
Yan, S. et al. Anti-Inflammatory Effect of Curcumin on the Mouse Model of Myocardial Infarction through Regulating Macrophage Polarization. Mediators Inflamm. 2021, 9976912 (2021).
-
Yue, L. et al. Chemotaxis-guided Self-propelled Macrophage Motor for Targeted Treatment of Acute Pneumonia. Adv. Mater. 35, e2211626 (2023).
-
Zhou, Y. et al. Curcumin Modulates Macrophage Polarization Through the Inhibition of the Toll-Like Receptor 4 Expression and its Signaling Pathways. Cell. Physiol. Biochem.: Int. J. Exp. Cell. Physiol., Biochem., Pharmacol. 36, 631–641 (2015).
-
Valizadeh, H. et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 89, 107088 (2020).
-
Fang, R. H., Kroll, A. V., Gao, W. & Zhang, L. Cell Membrane Coating Nanotechnology. Adv. Mater. 30, e1706759 (2018).
-
Krishnan, N. et al. A modular approach to enhancing cell membrane-coated nanoparticle functionality using genetic engineering. Nat. Nanotechnol. 19, 345–353 (2024).
-
Zhu, J. et al. Multimodal nanoimmunotherapy engages neutrophils to eliminate Staphylococcus aureus infections. Nat. Nanotechnol. 19, 1032–1043 (2024).
-
Yang, W. et al. An Engineered Bionic Nanoparticle Sponge as a Cytokine Trap and Reactive Oxygen Species Scavenger to Relieve Disc Degeneration and Discogenic Pain. ACS nano 18, 3053–3072 (2024).
-
Thamphiwatana, S. et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc. Natl. Acad. Sci. USA 114, 11488–11493 (2017).
-
Gao, C. et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 11, 2622 (2020).
-
Rao, L. et al. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc. Natl. Acad. Sci. USA 117, 27141–27147 (2020).
-
Wang, Z. et al. Inhaled ACE2-engineered microfluidic microsphere for intratracheal neutralization of COVID-19 and calming of the cytokine storm. Matter 5, 336–362 (2022).
-
Zhang, F. et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat. Mater. 21, 1324–1332 (2022).
-
Ou, J. et al. Micro-/Nanomotors toward Biomedical Applications: The Recent Progress in Biocompatibility. Small 16, e1906184 (2020).
-
Chen, W. et al. Recent Progress of Micro/Nanorobots for Cell Delivery and Manipulation. Adv. Funct. Mater. 32, 2110625 (2022).
-
Li, J., Esteban-Fernández de Ávila, B., Gao, W., Zhang, L. & Wang, J. Micro/Nanorobots for Biomedicine: Delivery, Surgery, Sensing, and Detoxification. Sci. Robot. 2, eaam6431 (2017).
-
Gao, C. et al. Biomedical Micro-/Nanomotors: From Overcoming Biological Barriers to In Vivo Imaging. Adv. Mater. 33, e2000512 (2021).
-
Xu, W. et al. Self-propelled ferroptosis nanoinducer for enhanced cancer therapy. Int. J. Extrem. Manuf. 7, 035501 (2025).
-
Huang, W. et al. Self-Propelled Proteomotors with Active Cell-Free mtDNA Clearance for Enhanced Therapy of Sepsis-Associated Acute Lung Injury. Adv. Sci. 10, e2301635 (2023).
-
Xu, C. et al. Magnesium-Based Micromotors as Hydrogen Generators for Precise Rheumatoid Arthritis Therapy. Nano Lett. 21, 1982–1991 (2021).
-
Xu, C. et al. Arthritic Microenvironment Actuated Nanomotors for Active Rheumatoid Arthritis Therapy. Adv. Sci. 10, e2204881 (2023).
-
Wu, Z. et al. A microrobotic system guided by photoacoustic computed tomography for targeted navigation in intestines in vivo. Sci. Robot. 4, eaax0613 (2019).
-
Esteban-Fernández de Ávila, B. et al. Multicompartment Tubular Micromotors Toward Enhanced Localized Active Delivery. Adv. Mater. 32, e2000091 (2020).
-
Zhang, B. et al. Twin-bioengine self-adaptive micro/nanorobots using enzyme actuation and macrophage relay for gastrointestinal inflammation therapy. Sci. Adv. 9, eadc8978 (2023).
-
Zhang, L. et al. A Dual-Biomineralized Yeast Micro-/Nanorobot with Self-Driving Penetration for Gastritis Therapy and Motility Recovery. ACS nano 17, 6410–6422 (2023).
-
Huang, G. et al. Cell-based intelligent micro/nanorobots for precise regulation and active biotherapy. Matter 6, 4158–4194 (2023).
-
Zhang, F. et al. Biohybrid Microalgae Robots: Design, Fabrication, Materials, and Applications. Adv. Mater. 36, 2303714 (2024).
-
Zhang, H. et al. Dual-responsive biohybrid neutrobots for active target delivery. Sci. Robot. 6, eaaz9519 (2021).
-
Tang, X. et al. Magnetic-Acoustic Sequentially Actuated CAR T Cell Microrobots for Precision Navigation and In Situ Antitumor Immunoactivation. Adv. Mater. 35, e2211509 (2023).
-
Gwisai, T. et al. Magnetic torque-driven living microrobots for increased tumor infiltration. Sci. Robot. 7, eabo0665 (2022).
-
Xing, J. et al. Sequential Magneto-Actuated and Optics-Triggered Biomicrorobots for Targeted Cancer Therapy. Adv. Funct. Mater. 31, 2008262 (2021).
-
Li, Q. et al. Nanoparticle-Regulated Semiartificial Magnetotactic Bacteria with Tunable Magnetic Moment and Magnetic Sensitivity. Small 15, e1900427 (2019).
-
Mei, Y. et al. Versatile Approach for Integrative and Functionalized Tubes by Strain Engineering of Nanomembranes on Polymers. Adv. Mater. 20, 4085–4090 (2008).
-
Liu, L. et al. Magnetically Actuated Biohybrid Microswimmers for Precise Photothermal Muscle Contraction. ACS nano 16, 6515–6526 (2022).
-
Ortiz-Muñoz, G. & Looney, M. R. Non-invasive Intratracheal Instillation in Mice. Bio-Protoc. 5, e1504 (2015).
-
Liu, K. et al. Clinical HDAC Inhibitors Are Effective Drugs to Prevent the Entry of SARS-CoV2. ACS Pharmacol. Transl. Sci. 3, 1361–1370 (2020).
-
Zhong, D. et al. Orally deliverable strategy based on microalgal biomass for intestinal disease treatment. Sci. Adv. 7, eabi9265 (2021).
-
Yuan, R. et al. Fe-Curcumin Nanozyme-Mediated Reactive Oxygen Species Scavenging and Anti-Inflammation for Acute Lung Injury. ACS Cent. Sci. 8, 10–21 (2022).
-
Mikawa, K., Nishina, K., Takao, Y. & Obara, H. ONO-1714, a nitric oxide synthase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesthesia analgesia 97, 1751–1755 (2003).
-
Parodi, A. et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 8, 61–68 (2013).
-
Chen, Z. et al. Cancer Cell Membrane-Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS nano 10, 10049–10057 (2016).
-
Zhang, L. et al. Cancer-macrophage hybrid membrane-camouflaged photochlor for enhanced sonodynamic therapy against triple-negative breast cancer. Nano Res. 15, 4224–4232 (2022).
-
Zhang, L. et al. A NIR-driven green affording-oxygen microrobot for targeted photodynamic therapy of tumors. Nanoscale 16, 635–644 (2024).
-
Yang, C., Takeyama, H., Tanaka, T. & Matsunaga, T. Effects of growth medium composition, iron sources and atmospheric oxygen concentrations on production of luciferase-bacterial magnetic particle complex by a recombinant Magnetospirillum magneticum AMB-1. Enzym. Microb. Technol. 29, 13–19 (2001).
Acknowledgements
This work was supported by National Key Research and Development Program of China (2022YFA1206900, Y.T.), National Natural Science Foundation of China (22175083, Y.T. and 22375224, F.P.), Guangdong Basic and Applied Basic Research Foundation (2023A1515110932, W.H.), and Dongguan Social Development Science and Technology Project (20221800906292, L.S. and 20211800904722, W. H.).
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks anonymous reviewers for their contribution to the peer review of this work. [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Chen, Z., Ran, H. et al. Biomimetic magnetobacterial microrobots for active pneumonia therapy. Nat Commun 16, 7856 (2025). https://doi.org/10.1038/s41467-025-63231-6
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41467-025-63231-6