Introduction
Hair follicles undergo a lifelong cyclic regeneration known as the hair cycle, a combination of the anagen (rapid growth), catagen (hair regression), and telogen (quiescence) phases1. Hair cycle abnormalities cause hair disorders, including alopecia; treating such disorders necessitates a clear understanding of hair-cycle maintenance mechanisms. Dermal papilla (DP) produce growth factors that regulate hair growth/regression2. Multiple variables (including inflammation3, hormones4, stress5,6, vitamins7, and minerals7) influence DP action.
Recent studies have highlighted the effects of hair-intestine interactions on hair follicle homeostasis. The intestines contain a symbiotic microbiome and specialized cells that produce factors implicated in hair growth/regression8. For instance, the water-soluble vitamin biotin (B7) is highly dependent on bacterial production and essential for skin health. Biotin deficiency is associated with severe dermatological conditions, including alopecia9. A recent mouse study showed that Lactobacillus murinus overgrowth induced alopecia via decreasing biotin production10. Another metabolite produced by the gut microbiome is equol, derived from soy isoflavones and effective against hair loss11. Equol exerts anti-aging properties on hair follicles in postmenopausal women, promoting better hair manageability, bounce, shine, and luster12. Although the causal compound is unknown, Lactobacillus paracasei HY7015 increases serum growth factor levels to induce DP cell proliferation, in turn promoting hair growth13. Understanding the relationship between the intestinal microbiome and hair follicles would benefit the development of new alopecia treatments.
The neurotransmitter serotonin, also known as 5-hydroxytryptamine (5-HT), is synthesized in the raphe nuclei of the brainstem and enterochromaffin cells of the intestinal mucosa14,15. Circulating serotonin is mainly produced via intestine-microbiome interactions16. Serotonin is a regulator of numerous physiological activities, including behavior, mood, memory, neurogenesis, embryonic development, smooth muscle homeostasis, liver homeostasis, and gastrointestinal homeostasis17,18,19. In hair follicles, 5-HT plays a role in regulating hair pigmentation and stress-induced depigmentation20, but its exact influence on hair growth/regression remains unclear.
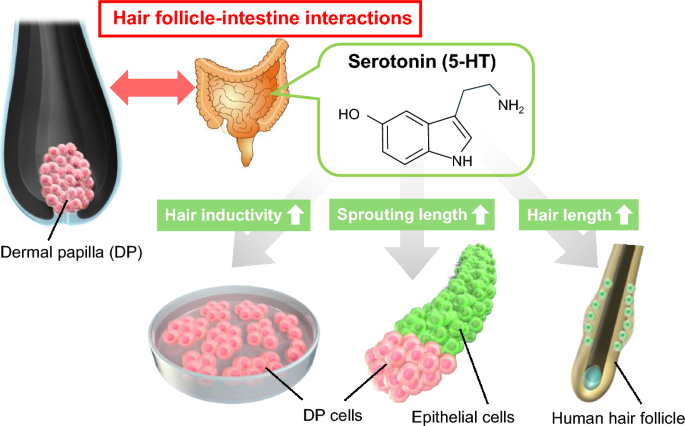
In this study, we investigated the effects of 5-HT on DP cells using 2D cultures, hair follicle organoids, and organ cultures (Fig. 1). We also analyzed the effects of 5-HT receptor (HTR) agonists on the promotion of hair growth. Our findings should provide insight into 5-HT involvement in the hair cycle and enhance understanding of the relationship between hair follicles and the intestines.
Scheme for understanding the effects of serotonin (5-hydroxytryptamine, 5-HT) on dermal papilla (DP) cells and hair growth. The effects of 5-HT were studied using human DP cells, hair follicloids, and hair follicles.
Results
Effects of 5-HT on DP cells
We added various concentrations of 5-HT to DP cells cultured in a 24-well plate (Fig. 2a). Hair growth-related genes, such as ALP and VEGFA, tended to increase with increasing 5-HT concentrations (Fig. 2b). RNA-seq analysis showed that 5-HT-treated-DP cells had significantly upregulated genes related to serotonergic synapses (Fig. 2c). These genes were associated with transcription factors for serotonin signaling and 5-HT receptors (Fig. 2c), suggesting that DP cells enhance serotonin signaling via increasing 5-HT receptors. Results from KEGG analysis also revealed enrichment of calcium and cAMP signaling pathways, second messengers of serotonin signaling21 (Fig. 2c, Supplemental Table 1). These results suggest that 5-HT upregulation of serotonin signaling promotes hair growth-related genes in DP cells.
Serotonin-induced changes in gene expression of DP cells (a) Procedures for testing the effects of 5-HT on cultured DP cells. (b) Expression of DP marker genes. GAPDH was used for normalization. Error bars represent the standard error of the mean calculated from three experiments for each condition. * indicates p < 0.05 (Tukey’s test). (c) The top 15 KEGG pathways enriched by upregulated DEGs from RNA-seq. 5-HT, 5-hydroxytryptamine; D, days; DP, Dermal papilla; RT-PCR, reverse transcription-polymerase chain reaction; KEGG, Kyoto Encyclopedia of Genes and Genomes; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; DEGs, differentially expressed genes.
Hair growth-promoting effects of 5-HT on hair follicloids
In our previous studies, we developed mouse hair follicle organoids (hair follicloids) by co-culturing two types of follicular stem cells in a low-concentration Matrigel-containing medium on spheroid-forming plates22. The two cell types formed core–shell-shaped aggregates after 2 days and generated mature hair follicles with nearly 100% efficiency in vitro after 10 days. After 23 days of culture, hair shaft elongation reached 3 mm. Applying this approach to human cells showed that immature hair shaft-like structures sprouted after 4 days of culture, and the length increased for at least 10 days23. The sprouting speed of hair shaft-like structures in hair follicloids increases in response to minoxidil, a typical hair growth-promoting agent23. Using human hair follicloids, we examined potential hair growth-promoting drug candidates and identified the effects of oxytocin, cinnamic acid, and LIT001 on hair growth24,25,26. In this study, we investigated the effects of 5-HT treatment on hair growth using hair follicloids (Fig. 3a). Human DP and epithelial cells were mixed with 2% v/v Matrigel-containing medium and seeded in 96-well spheroid formation plates. From days 4 to 10 of culture, 5-HT was added to the culture medium. The sprouting length of hair shaft-like structures significantly increased with 5-HT treatment (Fig. 3b, c), indicating that 5-HT promotes hair follicle-like growth.
Testing the effect of 5-HT on hair growth in hair follicloids. (a) Procedures for testing the effects of 5-HT on hair follicloids. (b) Microscope images of hair follicloids cultured with/without 5-HT for 10 days. Hair follicloids were observed under a stereomicroscope. (c) Length of sprouting structures with/without 5-HT. Length ratios on days 6, 8, and 10 are compared with the ratio on day 4. To calculate sprouting length, we cultured at least 38 hair follicloids per treatment condition. * indicates p < 0.05 (Tukey’s test). 5-HT, 5-hydroxytryptamine; D, days.
Effects of HTR agonist on hair growth promotion
Sumatriptan succinate, an HTR agonist, is clinically used to treat migraines27. We hypothesized that drug repositioning of existing HTR agonists may enable the development of hair growth-promoting drugs targeting serotonin. We investigated its effects on hair growth promotion using 2D cultures and hair follicloids (Fig. 4a). After treating DP cells with sumatriptan succinate for 3 days, and gene expression was analyzed using reverse transcription-polymerase chain reaction (RT-PCR) and RNA-seq. Hair growth-related genes, such as ALP and VEGF, showed increased expression with higher sumatriptan succinate concentrations (Fig. 4b). RNA-seq analysis showed that DP cells treated with 50 μM sumatriptan succinate significantly upregulated genes related to serotonergic synapse (Fig. 4c, Supplemental Table 2). In the hair follicloid assay, elongation of hair shaft-like structures was significantly increased by treatment with sumatriptan succinate (Fig. 4d, e). These results suggest that sumatriptan succinate promotes hair follicle-like growth via 5-HT signaling activation. Drug repositioning of existing HTR agonists may enable the development of hair growth-promoting drugs targeting serotonin.
Treatment with 5-HT receptor agonist (sumatriptan succinate). (a) Procedures for sumatriptan succinate treatment in DP cells and hair follicloids. (b) Expression of DP marker genes. GAPDH was used for normalization. Error bars represent the standard error of the mean calculated from three experiments per condition. * indicates p < 0.05 (Tukey’s test). (c) The top 15 KEGG pathways enriched by upregulated DEGs from RNA-seq. (d) Images of hair follicloids cultured with/without sumatriptan succinate for 10 days, observed under a stereomicroscope. (e) Length of sprouting structures with/without sumatriptan succinate, calculated from at least 48 hair follicloids per treatment condition. Length ratios on days 6, 8, and 10 are compared with the ratio on day 4. * indicates p < 0.05 (Student’s t-test). 5-HT, 5-hydroxytryptamine; D, days; RT-PCR, reverse transcription-polymerase chain reaction; KEGG, Kyoto Encyclopedia of Genes and Genomes; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; DEGs, differentially expressed genes.
Hair growth assay using human hair follicle organ culture
Human hair follicle organ culture is a reliable approach for understanding the effects of drugs in alopecia treatment, although considerable obstacles block easy access to fresh human hair follicles28. To investigate the effects of 5-HT in promoting hair growth, we treated human hair follicles with 100 µM 5-HT for 6 days (Fig. 5a). As expected, 5-HT treatment significantly promoted the elongation of human hair shafts (Fig. 5b, c).
Effects of 5-HT on hair shaft elongation in human hair follicle organ culture. (a) Schematic of the 5-HT treatment in hair follicles. (b) Microscopic images of hair follicles cultured with/without 5-HT for 6 days. (c) Length of sprouting structures with/without 5-HT. Length ratios on days 3 and 6 are compared with the ratio on day 0. Scalp hair follicles were extracted from patients with AGA and examined in 24-well cell culture plates. Error bars represent the standard divisions calculated from three independent experiments. *p < 0.05 (Student’s t-test). 5-HT, 5-hydroxytryptamine; D, days.
Discussion
Serotonin is mainly produced in the intestines and circulates throughout the body via the bloodstream. To the best of our knowledge, this is the first study demonstrating that 5-HT stimulates DP cells to promote hair growth. We found that the expression levels of hair growth-promoting factors increased via activation of serotonin signals in DP cells. Outcomes from both hair follicloid assays and human hair follicle organ cultures supported the hair growth-promoting effects of 5-HT. This finding may improve our understanding of the mechanism involved in maintaining hair follicle homeostasis, specifically via hair follicle-intestine interactions.
Notably, serotonin is produced by outer root sheath (ORS) cells upon mechanical stimulation29. The serotonin secreted from ORS cells may be a signal that mediates epithelial-mesenchymal interaction for hair growth. Coculture experiments of DP and ORS cells that inhibited/activated serotonin secretions may elucidate the relationship between mechanical stimulation and hair growth.
Multiple circulating hormones influence DP cells30. For example, dihydrotestosterone shortens the anagen phase of DP cells31, while increased estrogen levels (e.g., during pregnancy) spurs additional hair growth30. Likewise, oxytocin and cortisol exert opposing effects on DP cells and hair growth, the former activating growth factor secretion (stimulatory)26 and the latter decreasing Gas6 expression (inhibitory)32. The circadian hormone melatonin promotes hair regeneration via activating Wnt signaling in DP cells33. Research on novel hair growth-promoting hormones has accelerated worldwide in pharmaceutical and cosmetic industries, largely targeting these hormones. Here, we demonstrated that 5-HT promotes hair growth in addition its known effects on mood regulation. Future studies should investigate hair growth mechanisms and side effects in both human hair follicles and mouse models. For instance, one promising topic of research is to investigate whether feeding tryptophan (5-HT precursor) to mice will promote hair growth. Another promising topic of research is transdermal administration (e.g., on alopecia model mice), given that the low molecular weight (176.21 g/mol) of 5-HT allows for ready absorption. The concentration and duration of treatment can then be precisely optimized in such experiments. These studies would verify 5-HT suitability as a potential drug candidate for treating alopecia.
The transcriptome analysis of the DP cells revealed that 5-HT treatment promoted the cAMP signaling pathway (Fig. 2c), and dermal papilla cells upregulated VEGF expression after 5-HT treatment (Fig. 2b). Previous studies have shown that cAMP signaling promotes VEGF expression and secretion34. Based on these findings, one possible mechanism is that 5-HT treatment activates the cAMP signaling pathway to increase the production of hair growth promoting factors, including VEGF. However, 5-HT promotes hair growth via several possible mechanisms, and further studies are required to confirm this.
Previous studies have shown that minoxidil increases the expression of genes related to hair growth-promoting factors such as FGF735, while oxytocin and oxytocin receptor agonists increase VCAN and LEF1 expression24,26. We analyzed the expression of these genes, but did not find any significant upregulation after 5-HT treatment (Supplemental Fig. 1). If 5-HT promotes hair growth via pathways other than those of minoxidil and oxytocin, combined modulation of these pathways may synergically activate hair growth. Comprehensive gene expression analysis using 5-HT treated DP cells from patients with alopecia (of different ages and sexes) may elucidate the 5-HT-induced hair growth mechanisms. Hair growth should also be investigated through percutaneous absorption to apply a combination of serotonin, minoxidil, and oxytocin to an alopecia mouse model. These two investigations combined will confirm the feasibility of a multi-pathway approach to hair loss therapy.
Physiological serotonin levels in the epidermis are ~ 1 μM orders of magnitude lower than the effect seen in this study. Serotonin treatment at high concentrations may cause off-target effects in the body. Serotonin syndrome, caused by an overdose of serotonin, can trigger psychiatric, neuromuscular, and autonomic symptoms. Considering the clinical application of serotonin signal activation for hair loss treatment, investigation of serotonin alternatives with lower off-target effects may be necessary. Several studies have reported that selective serotonin reuptake inhibitors (SSRIs) can lead to drug-induced alopecia36,37. The discovery of serotonin alternatives that are not SSRIs will encourage the development of new hair growth-promoting drugs.
Serotonin initiates signal transduction via binding to G protein-coupled HTRs38. Here we demonstrated the hair growth-promoting effects of sumatriptan succinate, which targeted HTR1B and HTR1D (Fig. 4). Although we investigated only one agonist, other serotonin receptor subtypes (including HTR1, HTR2, HTR3, HTR4, HTR5, HTR6, and HTR7) may be present on the DP cell membrane18. Western blotting and immunostaining of HTR subtypes will help us understand which ones are expressed on DP cells and therefore which can be applied to promote hair growth. In addition, an increase in serotonin receptors themselves is also effective in 5-HT signal activation. Thus, serotonin-receptor-boosting substances such as caffeine39 may activate 5-HT signaling and promote hair growth.
In conclusion, we demonstrated that 5-HT and HTR agonist activate DP cells and promote hair growth. These findings encourage further research on the clinical application of 5-HT therapy in patients with hair loss.
Methods
Human DP and epithelial cells
Human DP cells were obtained from PromoCell (Heidelberg, Germany), subcultured up to passage four in DP cell growth medium (DPCGM; PromoCell), and used for 2D and hair follicloid cultures. Human follicular keratinocytes were obtained from Scientific Cell Research Laboratories (Carlsbad, CA, USA). Passage-four DP cells and passage-one keratinocytes were used for hair follicloid culture. Cells were cultured in a humified 5% CO2 incubator at 37 °C.
Serotonin and 5-HT receptor agonist treatment of DP cells
A suspension of DP cells (2 × 104 cells) in 0.5 mL DPCGM was seeded into a 24-well culture plate (Corning Inc., Corning, NY, USA). After 1 day of culture, the medium was exchanged to fresh DPCGM supplemented with either 0, 12, 25, 50, 100, or 200 μM 5-HT (Sigma-Aldrich, St. Louis, MO, USA) or 0, 12, 25, or 50 μM HTR agonist (sumatriptan succinate; Abcam, Cambridge, UK). Cellular gene expression was assessed using real-time PCR after 3 days of culture.
Gene expression analysis
Total RNA was extracted from samples using RNeasy Mini Kit (Qiagen, Hilden, Germany) and used for complementary DNA synthesis using the ReverTra Ace® quantitative RT-PCR Kit (Toyobo, Osaka, Japan). Subsequent quantitative reverse transcriptase PCRs were performed using the StepOne Plus RT-PCR system (Applied Biosystems, Waltham, MA, USA) with SYBR® Premix Ex Taq™ II (Takara Bio, Kusatsu, Japan) and primers for amplifying human ALP, VEGFA, and the reference gene GAPDH (see Table 1). The 2−∆∆Ct method was used to determine relative gene expression, presented as the mean ± standard error of 3 independent experiments. Differences in numerical variables were evaluated using Tukey’s test. Significance was set at p < 0.05.
RNA-seq analysis
Total RNA was extracted from DP cells with/without 100 μM 5-HT or 50 μM Sumatriptan succinate treatment using an RNeasy Mini Kit (Qiagen). RNA-seq analysis was performed by Takara Bio. Differentially upregulated genes were subjected to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses using the Database for Annotation, Visualization, and Integrated Discovery (http://david.abcc.ncifcrf.gov/)40,41. During analysis, differentially upregulated genes in the experimental groups versus control groups were selected (Supplemental Fig. 2). The 15 enriched terms according to the KEGG pathway analyses were ordered by “gene number” and exhibited significant P values.
Hair follicloid sprouting assay
To investigate the effects of 5-HT and 5-HT receptor agonists on hair growth, 5 × 103 each of DP and epithelial cells were suspended in 0.2 mL advanced Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12; Thermo Fisher Scientific) containing 2% (v/v) Matrigel (Corning) and seeded in a non-cell-adhesive round-bottom 96-well plate (Primesurface® 96U plate; Sumitomo Bakelite, Japan). DMEM/F-12 medium was supplemented with 100 μM 5-HT or 50 μM sumatriptan succinate for 4–10 days after seeding. Every 2 days subsequently, 0.1 mL of the spent medium was replaced with fresh medium. Hair sprout length was observed using an all-in-one fluorescence microscope (BZ-X810; Keyence). We determined the average length of sprouting structures using at least 38 samples.
Hair follicle organ culture
After informed consent was obtained, scalp hair follicles were obtained from patients with androgenic alopecia (AGA). This study was approved by the ethics committees of the Kanagawa Institute of Industrial Science and Technology (approval number: S-2019–01) and Yokohama National University (approval number: 2021–04). All procedures were in accordance with the guidelines of the Declaration of Helsinki. Extracted follicles were placed in 24-well cell culture inserts and cultured in DMEM/F-12 containing penicillin/streptomycin, either with or without 100 μM 5-HT. The length of black hair follicles was measured in ImageJ. We determined the average length of hair follicles using 3 samples.
Statistics and reproducibility
Statistical analyses of gene expression and hair sprout length were conducted using Tukey’s test or Student’s t-test. Significance was set at p < 0.05. All data are presented as mean ± standard error or standard division.
Data availability
The datasets generated and analyzed in this study are available in the NCBI repository GSE291220 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE291220). Enter token “gfmxcccgfvodtav” into the box. Further queries are directed to the corresponding author.
References
-
Oh, J. W. et al. A guide to studying human hair follicle cycling in vivo. J. Invest. Dermatol. 136, 34–44. https://doi.org/10.1038/JID.2015.354 (2016).
-
Legué, E. & Nicolas, J. F. Hair follicle renewal: Organization of stem cells in the matrix and the role of stereotyped lineages and behaviors. Development 132, 4143–4154. https://doi.org/10.1242/dev.01975 (2005).
-
Saceda-Corralo, D. et al. Association of inflammation with progression of hair loss in women with frontal fibrosing alopecia. JAMA Dermatol. 156, 700–702. https://doi.org/10.1001/jamadermatol.2020.0359 (2020).
-
Hasan, R. et al. Effects of hormones and endocrine disorders on hair growth. Cureus 14, e32726. https://doi.org/10.7759/cureus.32726 (2022).
-
Arck, P. C. et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am. J. Pathol. 162, 803–814. https://doi.org/10.1016/S0002-9440(10)63877-1 (2003).
-
Hughes, E. C., Syed, H. A. & Saleh, D. Telogen Effluviumin. StatPearls. https://pubmed.ncbi.nlm.nih.gov/28613598/ (2025).
-
Almohanna, H. M., Ahmed, A. A., Tsatalis, J. P. & Tosti, A. The role of vitamins and minerals in hair loss: A review. Dermatol. Ther. (Heidelb) 9, 51–70. https://doi.org/10.1007/s13555-018-0278-6 (2019).
-
Mahmud, M. R. et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 14, 2096995. https://doi.org/10.1080/19490976.2022.2096995 (2022).
-
Mock, D. M. Skin manifestations of biotin deficiency. Semin. Dermatol. 10, 296–302 (1991).
-
Hayashi, A. et al. Intestinal dysbiosis and biotin deprivation induce alopecia through overgrowth of Lactobacillus murinus in mice. Cell Rep. 20, 1513–1524. https://doi.org/10.1016/j.celrep.2017.07.057 (2017).
-
Brotzu, G. et al. A liposome-based formulation containing equol, dihomo-gamma-linolenic acid and propionyl-l-carnitine to prevent and treat hair loss: A prospective investigation. Dermatol. Ther. 32, e12778. https://doi.org/10.1111/dth.12778 (2019).
-
Soh Iwashita, H. M., Ueno, T., Hamamoto, K., Uchiyama, S. & Ueki, R. Equol status affects hair aging in postmenopausal women: A cross-sectional study. J. Jpn. Soc. Aesthetic Dermatol. 30, 8–17 (2020).
-
Nam, W. et al. Lactobacillus paracasei HY7015 promotes hair growth in a telogenic mouse model. J. Med. Food 24, 741–748. https://doi.org/10.1089/jmf.2020.4860 (2021).
-
Hornung, J. P. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 26, 331–343. https://doi.org/10.1016/j.jchemneu.2003.10.002 (2003).
-
Raghupathi, R. et al. Identification of unique release kinetics of serotonin from guinea-pig and human enterochromaffin cells. J. Physiol. 591, 5959–5975. https://doi.org/10.1113/jphysiol.2013.259796 (2013).
-
Liu, N. et al. The mechanism of secretion and metabolism of gut-derived 5-hydroxytryptamine. Int. J. Mol. Sci. 22, 7931. https://doi.org/10.3390/ijms22157931 (2021).
-
Layunta, E. et al. Intestinal serotonergic system is modulated by toll-like receptor 9. J. Physiol. Biochem. 78, 689–701. https://doi.org/10.1007/s13105-022-00897-2 (2022).
-
Karmakar, S. & Lal, G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics 11, 5296–5312. https://doi.org/10.7150/thno.55986 (2021).
-
Bamalan, O. A., Moore, M. J. & Al Khalili, Y. Physiology, Serotoninin. StatPearls. https://pubmed.ncbi.nlm.nih.gov/31424752/ (2025).
-
Wu, H. L. et al. 5-HT1A/1B receptors as targets for optimizing pigmentary responses in C57BL/6 mouse skin to stress. PLoS ONE 9, e89663. https://doi.org/10.1371/journal.pone.0089663 (2014).
-
Amireault, P. & Dubé, F. Intracellular cAMP and calcium signaling by serotonin in mouse cumulus-oocyte complexes. Mol. Pharmacol. 68, 1678–1687. https://doi.org/10.1124/mol.104.010124 (2005).
-
Kageyama, T. et al. Reprogramming of three-dimensional microenvironments for in vitro hair follicle induction. Sci. Adv. 8, eadd4603. https://doi.org/10.1126/sciadv.add4603 (2022).
-
Kageyama, T., Miyata, H., Seo, J., Nanmo, A. & Fukuda, J. In vitro hair follicle growth model for drug testing. Sci. Rep. 13, 4847. https://doi.org/10.1038/s41598-023-31842-y (2023).
-
Kageyama, T., Seo, J., Yan, L. & Fukuda, J. Effects of oxytocin on the hair growth ability of dermal papilla cells. Sci. Rep. 13, 15587. https://doi.org/10.1038/s41598-023-40521-x (2023).
-
Kageyama, T., Seo, J., Yan, L. & Fukuda, J. Cinnamic acid promotes elongation of hair peg-like sprouting in hair follicle organoids via oxytocin receptor activation. Sci. Rep. 14, 4709. https://doi.org/10.1038/s41598-024-55377-y (2024).
-
Kageyama, T., Seo, J., Yan, L. & Fukuda, J. Effects of oxytocin receptor agonists on hair growth promotion. Sci. Rep. 14, 23935. https://doi.org/10.1038/s41598-024-74962-9 (2024).
-
Moskowitz, M. A. & Cutrer, F. M. Sumatriptan: A receptor-targeted treatment for migraine. Annu. Rev. Med. 44, 145–154. https://doi.org/10.1146/annurev.me.44.020193.001045 (1993).
-
Langan, E. A., Philpott, M. P., Kloepper, J. E. & Paus, R. Human hair follicle organ culture: Theory, application and perspectives. Exp. Dermatol. 24, 903–911. https://doi.org/10.1111/exd.12836 (2015).
-
Agramunt, J. et al. Mechanical stimulation of human hair follicle outer root sheath cultures activates adjacent sensory neurons. Sci. Adv. 9, eadh3273. https://doi.org/10.1126/sciadv.adh3273 (2023).
-
Grymowicz, M. et al. Hormonal effects on hair follicles. Int. J. Mol. Sci. 21, 5342. https://doi.org/10.3390/ijms21155342 (2020).
-
Nestor, M. S., Ablon, G., Gade, A., Han, H. & Fischer, D. L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 20, 3759–3781. https://doi.org/10.1111/jocd.14537 (2021).
-
Choi, S. et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 592, 428–432. https://doi.org/10.1038/s41586-021-03417-2 (2021).
-
Niu, Y. L. et al. Melatonin promotes hair regeneration by modulating the Wnt/beta-catenin signalling pathway. Cell Prolif. 57, e13656. https://doi.org/10.1111/cpr.13656 (2024).
-
Kang, W., Park, S., Choi, D., Son, B. & Park, T. Activation of cAMP signaling in response to alpha-phellandrene promotes vascular endothelial growth factor levels and proliferation in human dermal papilla cells. Int. J. Mol. Sci. 23, 8959. https://doi.org/10.3390/ijms23168959 (2022).
-
Lee, C. Y. et al. Hair growth is promoted by BeauTop via expression of EGF and FGF-7. Mol. Med. Rep. 17, 8047–8052. https://doi.org/10.3892/mmr.2018.8917 (2018).
-
Pejcic, A. V. & Paudel, V. Alopecia associated with the use of selective serotonin reuptake inhibitors: Systematic review. Psychiatry Res. 313, 114620. https://doi.org/10.1016/j.psychres.2022.114620 (2022).
-
Krasowska, D., Szymanek, M., Schwartz, R. A. & Myśliński, W. Cutaneous effects of the most commonly used antidepressant medication, the selective serotonin reuptake inhibitors. J. Am. Acad. Dermatol. 56, 848–853. https://doi.org/10.1016/j.jaad.2006.10.020 (2007).
-
McCorvy, J. D. & Roth, B. L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 150, 129–142. https://doi.org/10.1016/j.pharmthera.2015.01.009 (2015).
-
Kazunori Sasaki, A. K. O. & Isoda, H. Hair Growth-Promoting effect of the coffee bean residue extract on hair follicle dermal papilla cells via the activation of autophagy. J. Funct. Foods. https://doi.org/10.1016/j.jff.2024.106251 (2024).
-
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
-
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. https://doi.org/10.1093/nar/gkac963 (2023).
Acknowledgements
This work was partially supported by the Kanagawa Institute of Industrial Science and Technology (KISTEC), grants-in-aid for scientific research (KAKENHI; 23K13614 and 23H01771), the Japan Agency for Medical Research and Development (23bm1123031h0001), Takeda Science Foundation, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Funding
Japan Society for the Promotion of Science, 23K13614, 23H01771, Japan Agency for Medical Research and Development, 23bm1123031h0001, Takeda Science Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Kanagawa Institute of Industrial Science and Technology.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kageyama, T., Seo, J., Yan, L. et al. Serotonin activates dermal papilla cells and promotes hair growth. Sci Rep 15, 24525 (2025). https://doi.org/10.1038/s41598-025-10716-5
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-025-10716-5