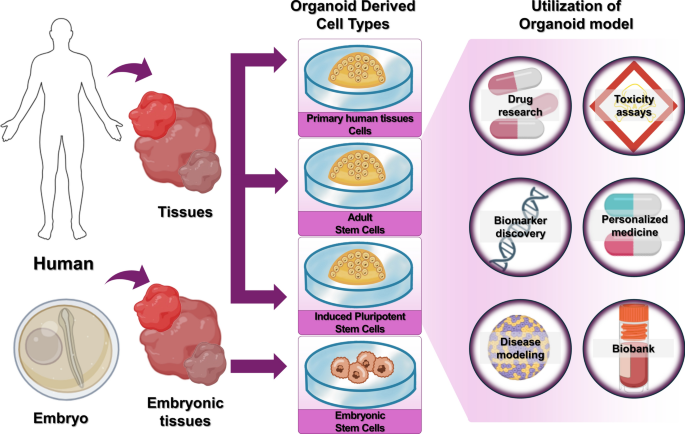
Organoid definition
Organoids are small 3D organ-like structures formed from embryonic stem cells (ESCs), adult stem cells (ASCs), or induced pluripotent stem cells (iPSCs) or primary human tissues through self-renewal, differentiation, and self-organization. The suffix ‘oid’ confers the meaning ‘similar to an organ’. Organoids contain various types of cells and closely mimic the structure and function of human tissues and organs. Organoids made from human tumor cells have histological, genetic, molecular, and other characteristics similar to those found in the original tumor. They are used in drug research, toxicity assays, biomarker discovery, personalized medicine, disease modeling, and biobanks (Fig. 1).
Types of organoids based on cell origin. Types of organoids include ASCs, iPSCs, and ESCs. These are used in drug research, toxicity assays, biomarker discovery, personalized medicine, disease modeling, and biobank.
Organoids derived from ESCs, ASCs, and iPSCs cells, and primary human tissues are 3D structures formed through processes such as self-renewal and differentiation15,16. The main application areas of organoids are disease models and regenerative therapy17. Simulating diseases such as cancer, dementia, and infection with cell-based organoid models can be used to study disease mechanisms instead of using humans or animals, and can also be used in the field of new drug development. Organoid disease models are being developed as an important test method that can reduce cost and time by verifying the performance of new drug candidates before clinical trials. Organoids mimic faithfully the human body, so they can be used to study various diseases that are difficult to study using existing animal disease models. When organoids are transplanted into damaged parts of an organ, they have a good ability to repair or replace damaged tissue. It is expected that this regenerative ability will be used to develop treatments for various incurable diseases.
Organoid-derived cell types and production methods
Organoids are manufactured by aggregating and recombining cells using 3D culture methods and contain specific cells that make up the modeled organ.
ESCs are extracted from embryos before differentiation. A blastocyst develops through the cell division of a fertilized egg, and an internal cell mass develops within the blastocyst. This mass gives rise to tissues such as blood, bone, and skin. ESCs can be isolated by separating internal mass cells and then differentiated into any type of cell or tissue by culturing them in vitro in a specific environment. Therefore, they are also called ‘pluripotent stem cells’18 (Supplementary Table S1).
ASCs go through an initial differentiation stage and differentiate only into cells with a defined function. They play a role in regenerating damaged tissue while maintaining the cellular characteristics of tissues or organs, e.g. when wounded skin heals and new skin grows. Representative ASCs are mesenchymal stem cells (MSCs)18. These cells are called”Adult Stem Cells”because they primarily function in tissue maintenance and regeneration in mature organisms. This terminology reflects their role in adult tissues, where they exhibit limited differentiation potential compared to ESCs but retain the ability to repair and regenerate specific tissues throughout life (Supplementary Table S1).
iPSCs are created by reprogramming mature somatic cells that have completed their growth and can no longer differentiate. These artificially created cells have the same function as ESCs. The advantage of iPSCs is that they can be obtained without using embryos, and since they are derived from mature cells, there is no problem with immune rejection18 (Supplementary Table S1).
Recently, primary human tissue cells derived directly from living tissues have also been attracting attention. These are cells derived directly from living tissues and maintain essential functional characteristics, and organoids produced based on these cells can maintain the structural and functional characteristics of the original tissues for a long time, and are widely used in various fields such as disease modeling, drug efficacy and toxicity evaluation, and regenerative medicine19.
For example, it has been shown that primary organoids can reproduce the tumorigenesis process in vitro through the introduction of oncogenes20, There is also a case that showed that human adaptive immune response can be reproduced in vitro using organoids derived from human tonsil tissue21.
Organoids are produced mainly in a bottom-up manner from ESCs, ASCs, or iPSCs through self-renewal, differentiation, and self-organization (Supplementary Table S2). The bottom-up method has the advantage of being able to imitate the fine and complex organ structures very similar to those of the human body, but its disadvantage is the need to precisely control differentiation. This method sequentially creates the cells that make up the organ from a single stem cell.
The tissues and organs of our body are not simply a collection of cells, but rather a complex assembly of various substances surrounding the cells. This substance, called the extracellular matrix (ECM), plays an important role in addition to providing structural support.
The ECM not only serves as a support for cell attachment, but also transmits essential biochemical signals. It regulates important physiological processes such as cell proliferation, migration, differentiation, survival, and tissue structure maintenance22.
The composition of the ECM is slightly different for each organ, but it is composed of components such as fibrous proteins, proteoglycans, and glycosaminoglycans. These components are intertwined to form a complex three-dimensional network. This structure is both strong and flexible, allowing cells to function stably.
In addition to supporting cells, ECM also transmits signals to cells that determine the attachment location, migration path, growth rate, and differentiation direction of cells. In other words, ECM acts as a signal plate that guides cells to behave. ECM stores and releases signaling substances such as growth factors to regulate cell growth. Therefore, it is important to create an environment similar to ECM when growing cells in the laboratory23.Organoids are generally cultured in hydrated polymer 3D hydrogel systems, and are formed through stem cell proliferation and self-organization within a 3D structure composed of ECM-derived proteins24. These hydrogels can have various levels of elasticity, topographic permeability, and biodegradability, which greatly affect cell fate and function. ECM-based hydrogels are frequently utilized for organoid culture, with Matrigel in particular being widely used as the “golden standard” material to date23.
Since recently, top-down methods are also being actively explored. A top-down method creates organ analogs by 3D printing of cells or tissues that have already differentiated into various functional cell types. This has the advantage of using differentiated cells or tissue-extracted cells, making it easier to ensure reproducibility and produce uniform organ replicas. The top-down organoid manufacturing uses a 3D bioprinter to connect various cells that make up the target organ and to create organoids with specific functions. The organ’s constituent cells are distributed in layers to create a shape similar to that of the target organ, co-cultured, and several cells together with the extracellular matrix are injected into a pre-designed scaffold or a bioprinted structure to mimic and function as the target organ. The advantage of this method is that it is highly reproducible because it is based on dividing, connecting, and culturing cells that have already differentiated into functional cells25.
Advantages and disadvantages of 2D cell culture, 3D cell culture, and animal models
Cell culture research has mainly been conducted in a 2D cell culture environment. The problem with this environment is that the cells do not grow properly or their unique functions in the actual cell environment in the body are poorly reproduced. Due to these problems, 3D cell culture is receiving new attention. Its biggest advantage is that it can provide a 3D environment similar to that in the body, where the activity of cells is similar to that in vivo. This approach allows to apply biochemical and cell biological analysis techniques based on existing simple and efficient 2D cell culture to 3D cell culture and to overcome the limitations of 2D cell culture (Supplementary Table S3).
Types of infectious disease organoids
Infectious disease refers to “a disease that is caused by a specific pathogen or toxic substance of a pathogen and is spread from a person, animal, or other host infected with a pathogen or toxic substance to a susceptible host (human).”26 Before testing treatments for infectious diseases on humans, organoid models are manufactured and studied in vitro. Infectious disease models that rely mainly on the intestine, lung, and liver organoids are being actively used. Due to the recent coronavirus pandemic, lung, blood vessel, and kidney organoid models are also being actively used (Fig. 2). Here we introduce organoid models of seven organs used in infectious disease research, classify them according to the cells used in each model, and introduce research trends that rely on these organoids.
Types of infectious disease organoids. Various types of infectious diseases occur in the brain, liver, lung, intestines, kidney, skin, and blood vessel. Organoids are being actively used to study these infectious diseases.
Brain
The brain is derived from the ectoderm, and brain organoids are being studied as an in vitro model for various diseases such as microcephaly. Because it is difficult to obtain brain cells for primary cell (PC) culture (PCC), most brain organoid models are developed, produced, and researched using iPSCs or ESCs. The brain is the most complex and irreplaceable organ in the human body and is virtually irreplaceable. In particular, no animal model capable of simulating the human cerebral cortex has been developed, so the development of a brain organoid model capable of simulating the cerebral cortex is urgently required. Zika virus (ZIKV), which infects the brain, is suspected of causing microcephaly in human fetuses27. Because of the species specificity and cell type specificity of ZIKV, it is difficult to study it using mouse models or immortalized cell lines. The mechanism of ZIKV infection and microcephaly induction has been revealed using an in vitro brain organoid model capable of simulating fetal brain development28,29,30 (Table 1).
Qian et al.28 used human iPSCs to create human brain organoids, which resemble the forebrain structure during early fetal development and exposed the organoids to ZIKV. ZIKV primarily infected neural progenitor cells within the organoid and induced their apoptosis, suggesting that a shrinkage of the nerve cell layer causes fetal microcephaly (Supplementary Fig. S1).
Additionally, microcephaly was modeled using cerebral organoids produced from iPSCs derived from skin fibroblasts of patients with microcephaly32. The neuroepithelial tissue in patient-derived organoids was smaller than that in control organoids, which were generated from iPSCs reprogrammed from healthy donor fibroblasts under identical differentiation conditions. Moreover, nerve growth in the patient-derived organoids was excessive. This study found that damage to early progenitor cells leads to premature and excessive neural differentiation. The mechanism of microcephaly development was identified as failure of normal growth and differentiation of progenitor cell populations, leading to small brain size and formation of a small skull.
Brain organoids have also been used to model infections beyond ZIKV, such as SARS-CoV-2-induced neuronal damage and tauopathy, and HIV-related neuroinflammation via infected microglia39,43. However, their lack of vasculature and immune cells limits the modeling of neuroimmune dynamics and blood–brain barrier (BBB) function28,32,33. In particular, the absence of microglia impairs antiviral signaling pathways, including Toll-like receptor 3 (TLR3) activation and cytokine responses33,43. Structural limitations are also evident, as prolonged culture without vasculature leads to necrotic cores due to inadequate oxygen and nutrient diffusion32. Nevertheless, brain organoids offer a useful platform for modeling virus-induced neural injury and screening for neuroprotective agents36,38.
Liver
Approximately 2 million people around the world die every year from liver disease. The liver is actively studied as an organ due to its high unmet medical needs and commercial research potential. The liver develops through complex interactions between endodermal and mesodermal cells78. Liver organoids are being used to study various diseases such as hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, and amebic liver abscess. Research on SARS-CoV-2 infection mechanisms is also being conducted using a co-culture model with the virus. The liver is prone to toxic injury, so toxicity evaluation studies using liver organoids are also being actively conducted. Liver organoids have been developed using various protocols based on iPSCs, PCs, or cell lines (Table 1).
Malaria is caused by protozoan parasites such as Plasmodium berghei (P. berghei), Plasmodium yoelii (P. yoelii), Plasmodium falciparum (P. falciparum), and Plasmodium vivax (P. vivax) which invade hepatocytes in the liver during their life cycle. Malaria infection organoids were produced from iPSCs for antimalarial drug screening46. The screening showed that Plasmodium-infected iHLCs were sensitive to atovaquone, and their sensitivity to primaquine was enhanced through chemical maturation. Amoebic liver abscess organoids were produced using cultures of liver sinusoidal endothelial cells and the cell lines Huh-7 (hepatoma cells) or Huh7-NTCP (hepatocytes)47. Huh7-NTCP cells stably express sodium taurocholate cotransporting polypeptide (NTCP), a receptor for HBV. An infection model was created by simultaneously co-culturing hepatocytes in collagen with Entamoeba histolytica (E. histolytica), a protozoan parasite that causes amebiasis, and HBV, which causes a chronic infection (Supplementary Fig. S2). This co-culture model can ultimately be used to study chronic inflammation associated with cirrhosis and liver cancer47,48.
Liver organoids have been used to model hepatotropic and parasitic infections, including HBV, HCV, Plasmodium, and Entamoeba histolytica35,44,46,47,48,50. And they can recapitulate NTCP-mediated HBV entry and viral replication. In contrast, these infection processes are difficult to reproduce in animal models or 2D culture systems35,44,50. Co-culture approaches have also facilitated studies of host–parasite interactions and hepatic injury46,47,48. However, most models lack bile ducts, liver sinusoidal endothelial cells (LSECs), and Kupffer cells, limiting the simulation of bile transport, vascular responses, and immune-mediated damage35,44,47. In addition, metabolic function and long-term viral persistence vary across models50. Liver organoids have structural limitations, such as the lack of vascular and immune components. However, they are utilized to develop antiviral and antiparasitic therapeutic strategies, as they replicate the pathophysiology of chronic liver infections more effectively than animal models or 2D culture model.
Large and small intestines
The large and small intestines are the most exposed organs to bacterial and viruses. Co-culture models have been developed for various pathogens, such as Salmonella enteritidis, and Escherichia coli. The intestinal organoid model is being actively used in studies on organoid transplantation and organoid therapeutics, and also as a toxicity evaluation model to check gastrointestinal side effects (diarrhea, abdominal pain) of various drugs79,80. Intestinal organoids can be cultured not only from iPSCs, ESCs, and ASCs, but also from human primary tissues obtained through minimally invasive procedures such as endoscopy. This enables the generation of patient-derived intestinal organoids, which enhances accessibility for personalized disease modeling and therapeutic screening62.
Patients infected with SARS-CoV-2 show intestinal symptoms such as abdominal pain and diarrhea52,81. Due to its species specificity, SARS-CoV-2 is difficult to study using animal models such as mice and monkeys. However, because intestinal organoids express SARS-CoV-2 receptors similar to those in the human body, the mechanisms that cause the above intestinal symptoms can be analyzed (Table 1).
In large intestine organoids, it was confirmed that intestinal cells express angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 receptor, capable of mediating SARS-CoV-2 infection52. Imatinib, mycophenolic acid, and quinacrine dihydrochloride inhibited SARS-CoV-2 infection in drug screening using this model (Supplementary Fig. S3).
Norovirus is a major pathogen that causes infectious acute enteritis. Norovirus organoids were produced by infecting intestinal epithelial organoids produced using ASCs derived from the human small intestine with norovirus57. Norovirus replicated efficiently in these organoids, and its growth was further enhanced by bile. These findings suggest that intestinal epithelial organoids may be a useful in vitro model for analyzing the effects of antiviral drugs against norovirus.
Intestinal organoids have been used to model infections by major enteric pathogens, including norovirus57,82, rotavirus60,61,83, SARS-CoV-256, Clostridioides difficile (C. difficile)53, and Shiga toxin-producing E. coli (STEC)54,55. These models replicate core features of the intestinal epithelium, such as crypt–villus architecture, apical–basal polarity, and mucus secretion, and express relevant pathogen receptors54,55,59. They support productive infection and facilitate studies of barrier disruption, cytokine responses, and host–pathogen interactions56,57,61. However, the absence of immune cells and commensal microbiota limits their ability to model inflammation and microbe–epithelium cross53,61. Toxin-induced responses can be partially recapitulated but fail to capture systemic effects such as neutrophil recruitment or enterotoxin-mediated inflammation53,62. Though limited in physiological complexity, intestinal organoids serve as an in vitro system for exploring host–pathogen interactions and evaluating targeted therapies for gut infections58,60.
Lung
The lung is a complex organ derived from the endoderm and mesoderm84. The lung is used as a model to analyze the effectiveness of treatments for congenital diseases. In 2021, an organoid disease model study succeeded in uncovering the mechanism of SARS-CoV-2 infection by producing lung organoids infected with SARS-CoV-2. Lung organoid models are also used to study the pathogenesis of tuberculosis, pneumonia, and hantavirus pulmonary syndrome, and are widely used in the study of various pathogens. In addition to producing lung organoids from iPSCs or ESCs, research is also being actively conducted to produce them by culturing cells obtained from lung and airway biopsies of clinical patients (Table 1).
Lung tissue models for Mycobacterium tuberculosis infection were constructed by co-culturing human lung fibroblasts expressing type 1 collagen on a porous membrane with uninfected primary human monocytes and primary human macrophages infected with Mycobacterium tuberculosis71. Then, human bronchial epithelial cells were spread on the collagen–fibroblast matrix and further cultured. This multicellular tissue was exposed to air, and further culturing initiated extracellular matrix protein production, mucus secretion, and epithelial layer stratification. This lung infection organoid model demonstrated physiological responses resembling those of the human lung. It induced macrophage aggregation and monocyte migration at the infection site, reproducing key features of granuloma formation seen in tuberculosis. This organoid model of lung infection successfully mimicked the early stages of human tuberculosis granuloma.
In vitro models mimicking Staphylococcus aureus necrotizing pneumonia were produced by mixing lung fibroblasts with collagen and culturing them in the insert within the Transwell system Afterwards, bronchial epithelial cells were added and the culture was exposed to air. S. aureus strains isolated from patients with severe pneumonia (including necrotic pneumonia and pulmonary empyema), were used to infect lung organoids, while bacterial supernatant and purified α-toxin were applied to evaluate their effects on the organoids. Significant α-toxin-mediated lung epithelial damage causes PVL-mediated neutrophil cytotoxicity and the release of toxin-mediated cytokines and chemokines from the lung epithelium, leading to inflammation73. Using a lung organoid model, it was confirmed that pneumonia isolates secrete high levels of α-toxin and PVL, causing inflammation. Toxin-mediated damage to infected lung organoids is inhibited by treatment with multi-specific intravenous immunoglobulin antibodies targeting α-toxin and PVL, which are present in supernatants of clinical isolates (Supplementary Fig. S4).
Andes virus (ANDV) causes hantavirus pulmonary syndrome. MRC-5 (a human lung fibroblast cell line) and 16HBE14 (a human bronchial epithelial layer cell line) were exposed to air to create a lung infection organoid model72. These organoids were infected with ANDV, and viral replication was detected about 1 week later. Viral replication was sustained for more than one week, as confirmed through quantitative analysis. This prolonged viral production was associated with increased levels of interferon lambda-1 (IFN-λ1), interferon lambda-2 (IFN-λ2), and interferon beta (IFNβ), along with elevated expression of the interferon-stimulated gene 56 (ISG56) mRNA. It was also cross-validated by examining whether the expression levels of pro-inflammatory cytokines interleukin 6 (IL-6) and interleukin 8 (IL-8) were increased in the Shiga toxin 2 (Stx2)-treated kidney organoid model. In this way, lung organoids enable long-term research on infectious diseases that is difficult to implement in monolayer cell culture models.
Lung organoids have been used to model infections by respiratory pathogens such as SARS-CoV-263,64,66,69,74,85, influenza virus65,66, Mycobacterium tuberculosis71, Staphylococcus aureus73, and hantavirus72. These models mimic key features of the airway epithelium under air–liquid interface conditions, including pseudostratification, mucus secretion, and cytokine release65,71,73. They enable analysis of viral tropism, innate immune responses, and tissue damage in a human-relevant setting63,66,74. However, most lung organoids lack vasculature and immune cells, limiting their ability to model systemic inflammation, immune infiltration, and vascular injury71,72,74. Alveolar differentiation also remains incomplete in some models, restricting the study of distal lung infections63,74. Despite these limitations, lung organoids serve as effective platforms for studying respiratory pathogens and evaluating targeted therapies69.
Kidney, skin, and blood vessels
The kidney is a representative mesoderm-derived organ, and kidney organoids are actively studied for their efficacy as in vitro models of various kidney diseases. Although kidney organoids can be obtained through PCC, most models use ESCs or iPSCs due to the complex structure of the glomerulus. For example, kidney organoids have provided new information about hemolytic uremic syndrome (HUS), which was the first evaluation of kidney damage caused by Stx that is characterized by renal failure, hemolytic anemia, and thrombocytopenia (Table 1).
Stx and its variants are bacterial cytotoxins secreted by Shigella dysenteriae (S. dysenteriae) and Stx-producing E. coli during intestinal infections. In the absence of lipopolysaccharide (LPS), Stx2 is toxic in both 2D and 3D cell culture systems. In particular, kidney organoids made from human cell lines showed have higher levels of kidney injury marker 1 (Kim-1) than cells cultured in a 2D system75. In a kidney organoid model treated with Stx2, secretion of inflammatory cytokines IL-6, IL-8, and tumor necrosis factor (TNF-α) was also found to increase.
Addition of human recombinant soluble ACE2 (hrsACE2) with SARS-CoV-2 inhibit the spread of SARS-CoV-269. The mechanism by which soluble ACE2 not only prevents lung damage but also blocks SARS-CoV-2 from entering target cells was confirmed in a kidney organoid model (Supplementary Fig. S5).
Kidney organoids have been used to model pathogen-induced renal injury, including SARS-CoV-2 infection69 and Shiga toxin–mediated cytotoxicity75. These models reproduce key features of proximal tubular damage and inflammatory cytokine responses. However, they lack vascular and immune components, limiting the study of systemic inflammation and vascular injury75. Additionally, incomplete nephron patterning constrains the modeling of complex renal architecture. While fully replicating the complexity of the human kidney remains difficult, kidney organoids prove studying nephrotoxicity and evaluating renal-targeted therapies75.
The skin is a multi-layered organ containing various components such as glands, hair follicles, temperature and pain nerves, and skin cells. Skin organoid models derived from iPSCs have limitations in mimicking aged skin. Nevertheless, skin organoids are widely used in infectious disease modeling and regenerative medicine (Table 1).
Bacterial and viral infection studies have been performed using human skin organoids76. It is difficult to conduct studies on virus latency and virus reactivation in monolayer cell cultures because these cultures lack the complex 3D tissue architecture and cellular interactions found in natural tissues. A skin organoid with a multi-layer structure has been created to confirm the infiltration of herpes simplex virus type 1 (HSV-1). Skin organoids composed of stratified epithelium and fibroblast dermal layer were used to study the pathogenesis of Streptococcus dysgalactiae subsp. equisimilis (SDSE)77. All SDSE strains can infect these organoids, and the bacteria look similar to those seen in tissue biopsies. An invasive SDSE strain spreads throughout the organoid epithelium, causing significant tissue damage through its destruction and separation from underlying tissue, which is similar to the infection symptoms and mechanisms in humans (Supplementary Fig. S6).
Skin organoids have been applied to model infections by HSV-1 and SDSE, recapitulating viral latency, bacterial invasion, and tissue injury76,77. These models reproduce stratified epidermal–dermal structure, enabling analysis of pathogen behavior in a physiologically relevant context. However, they lack immune components and features of aged skin, limiting their ability to model inflammatory responses and age-dependent vulnerability76,77. Even in the absence of immune elements and aging features, these skin organoids provide a valuable system for investigating localized infections and evaluating targeted treatments.
Blood vessels not only supply nutrients to organs, but also play an important role in the development, regeneration, and migration of brain nerve cells by providing a vascular microenvironment. Vascular organoids are used not only to simulate the path of infectious agents, but also to study the functions of specific proteins to prevent the spread of infectious diseases (Table 1).
Vascular organoids have recently been widely used to study SARS-CoV-2 infection. ACE2 is the main receptor for this infection. If a drug that inhibits the SARS-CoV-2–ACE2 interaction is developed, it is expected to be useful to treat COVID-19 patients A study using vascular organoids demonstrated that hrsACE2 binds to SARS-CoV-2 and reduces its entry into Vero cells by 1000–5000-fold, effectively blocking early infection69 (Supplementary Fig. S7).
Vascular organoids have been used to model endothelial infection by SARS-CoV-2 via ACE2, enabling evaluation of antiviral strategies such as soluble ACE2 treatment69. These models recapitulate key structural and functional features of human vasculature, including barrier properties and virus–receptor interactions. However, they lack immune cells and multicellular interactions with surrounding tissues, limiting their ability to simulate systemic inflammatory responses and inter-organ vascular effects. Although vascular organoid models have limitations, they provide a relevant platform for investigating vascular involvement in infectious diseases and evaluating targeted interventions.
Increasing importance of infectious diseases in the era of global environmental and societal change
In the twenty-first century, the emergence of various infectious diseases has significantly impacted human health and survival. In particular, the rapid spread of COVID-19 has posed serious challenges to society, the economy, and medical systems worldwide. Not only COVID-19, but also SARS-CoV in 2003, novel swine-origin influenza A (H1N1) virus in 2009, Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012, ebolavirus in West Africa from 2013 to 2016, and ZIKV infection in 2015 all resulted in high morbidity and mortality86,87. Infectious diseases can be caused not only by new viruses or bacteria but also by unicellular eukaryotic parasites and fungi, as well as by mutations or increased resistance of existing pathogens. Among infectious diseases, respiratory infections, diarrheal diseases, tuberculosis, AIDS, malaria, and Chagas disease can cause death88. Emerging infectious diseases such as COVID-19 and infections caused by antibiotic-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) are a threat. They now spread easily due to global factors such as climate change, population movement, and international trade. Accordingly, it is urgent to prepare countermeasures such as vaccines and treatments86,87.
Due to technological advancements that have led to the above global changes, the population continues to increase and climate is also changing. Infectious diseases easily spread across borders into many countries. Human health is closely connected to animals, which can serve as potential hosts of novel pathogens and the environment. As a result, it is tough to predict the emergence and decline of infectious diseases. Despite improvements in hygiene and access to health care worldwide, global changes are increasing the risk of infectious diseases86.
The continued existence of infectious disease-causing pathogens increases the need to understand the basic mechanisms of disease development and to develop treatments. Research on the onset and mechanisms of infectious diseases is difficult due to the complex conditions of pathogen cultures. Many pathogens do not infect non-human laboratory animals or do not interact with cell lines cultured in two dimensions because of their specificity to the host. As a result, animal and 2D cell culture models do not guarantee reliable results in pathogen modeling or drug testing within an appropriate time89,90. Long-term organoid platforms can solve these problems. Organoids have 3D structures and are composed of various cell types. They can reproduce physiological and pathological processes otherwise unique to human tissue91. Stem cells go through stages of self-renewal, differentiation, and self-organization under certain conditions. These stages are patterned and rearranged to have structures and functions similar to those of human organs15,16. Therefore, organoid models are more complex than 2D monolayer cell cultures and have a tissue-like structure. To effectively utilize organoids in infectious disease research, various engineered strategies have been developed, including 3D-embedded culture, air–liquid interface (ALI) culture, and organ-on-a-chip (Table 2). The advantages and limitations of these strategies in organoid-based infectious disease models are summarized in Table 2.
Organoids are used as essential models in the research and development to treat new infectious diseases as they can provide fast and reliable information about pathogen biology and be used for drug screening93 (Table 3).
There are three main types of organoid models for infectious diseases, which are specifically designed to model infectious diseases caused by viruses, bacteria, and protozoa98.