Introduction
Optical microscopy is an essential tool in biological research and a wide range of clinical applications including whole slide histopathological analysis1,2, quantitative fluorescence imaging3,4 and several neuroimaging applications5,6. In widefield microscopy, illumination uniformity is essential for high quality imaging and downstream image analysis. Non-uniform illumination, typically caused by vignetting7,8, compromises image quality and adversely affects subsequent qualitative and quantitative analysis. For example, qualitative interpretation can suffer from uneven contrast, while quantitative measurements may be skewed by spatial intensity variations. Light emitting diodes (LEDs) can serve as high-power sources for most forms of widefield microscopy; however, LEDs supply a variable radiance profile, resulting in a Gaussian-like intensity distribution even under Köhler illumination. While various optical9,10,11,12,13,14 and computational correction methods7,15,16,17,18 have been developed to address this issue, these techniques are not universally applicable, with each approach having specific limitations in terms of effectiveness, complexity, computational requirements, or cost. Thus, finding a robust, low-cost and widely adaptable solution for uniform illumination remains a challenge in optical microscopy.
Whole slide imaging (WSI) is a prime example, where illumination uniformity is vital for downstream analysis such as automated segmentation15,19,20, cell tracking algorithms17 or mosaic stitching18,21. WSI is commonly used in histopathological analysis to examine stained biological specimens for disease diagnosis1, including applications in any research discipline requiring high-throughput imaging2,22. With WSI, a series of images acquired across the entire sample are stitched together (tiled); however, shading and vignetting artifacts can significantly degrade seamless stitching and compromise analysis of spatial and intensity information in mosaic images. This is a significant issue for most forms of optical microscopy that employ tiling for large area scanning of tissue specimens at high local resolution. Computational correction techniques, such as BaSiC16 and CIDRE15, can be used to ameliorate tiling artifacts, but these techniques are computationally expensive, require many images for accurate correction, and, importantly, still suffer from reduced signal-to-noise ratio (SNR) in regions of lower intensity. These limitations highlight the need for improved illumination strategies, particularly for applications requiring high-throughput and precise quantitative analysis.
Quantitative imaging techniques are particularly sensitive to non-uniform illumination, which corrupts the collected data in ways that are difficult (or practically infeasible) to be corrected for computationally. For instance, in quantitative fluorescence imaging, the presence of irregularities in the spatial distribution of the excitation light can lead to non-uniform photobleaching, limiting the potential for longitudinal studies11. Similarly, neuroimaging and optogenetics are used for structural and functional imaging of complex response of cellular activities and structure, where computational corrections may not be applicable or desired23. Non-uniformity of illumination also affects other quantitative imaging methods, such as quantitative phase imaging24, spatial light interference microscopy (SLIM)25,26, and multispectral imaging microscopy27, by introducing variations in signal strength that degrade measurement accuracy. This is also true for label-free polarized light imaging (PLI) techniques, including Mueller matrix polarimetry10, PLI28,29,30, and birefringence microscopy31,32,33, which are used for quantitative structural imaging of birefringent specimens. In these types of microscopic techniques, non-uniform illumination leads to a spatially varying signal-to-noise ratio, which cannot be corrected computationally10. Ultimately, such non-uniformity creates unequal noise distribution across the imaging field of view (FOV), effectively limiting the dynamic range of the measurements and impacting quantitative analysis34. Lastly, molecular biosensors, such as the interferometric reflectance imaging sensor (IRIS), rely on uniform illumination for precise detection of local reflectance changes at multiple wavelengths for measuring analyte binding14,35. This highlights the need for a uniform illuminator that can provide high radiance over a large field-of-view for accurate and sensitive measurements across a wide array of quantitative imaging modalities.
Light distribution in an imaging system depends on the emission properties of the employed light source, as well as the optical elements that couple the light from the source to the object and from the object to the imaging sensor. Since the performance of a typical imaging system is determined by the image sensor (camera), the uniformity of light distribution is quantified based on the image acquired when an optically flat object is studied, rather than quantifying the field uniformity at the object. The uniformity on the camera depends on the optical transfer function of the imaging system, and it is desirable to have adjustable field distribution. Although constant radiance light sources can be used as uniform illuminators, such as integrating spheres, they cannot perform well when the illumination is coupled by lenses, common denominator of most microscopy configurations14. In many uniform illumination setups, the light source is corrected using either single-element or multi-element illumination systems9,10,13,36. Basic single-element collection methods include diffuser-coated lenses, light-homogenizing rods, or light guides. These methods randomize the input light via diffusive transmission or multiple total internal reflections. However, as demonstrated in this paper, they fail to provide sufficient uniformity and convenient adjustability for quantitative microscopy techniques that require high sensitivity to low-contrast signals in brightfield illumination or to high-dynamic-range signal differences. Illumination systems incorporating multiple optical components achieve improved uniformity because they are optimized for the specific imaging system in use. Examples include raster scanning and randomized illumination techniques. Raster scanning involves precisely steering a focused beam across the sample within the sensor’s exposure time13. This approach, commonly demonstrated using laser light, is effective but generally limited to narrow fields of view. It also requires sophisticated optics, is time-inefficient for widefield imaging, and introduces speckle artifacts37. On the other hand, randomized illumination techniques, using diffusers to randomize the input beam and tandem lenses, are applicable to both coherent and incoherent light sources36,38,39. In these methods, the input light first passes through a transparent diffuser, which scatters the beam and generates numerous new point sources. These point sources are then imaged onto the sample using two tandem microlenslet arrays. This design demonstrates improved accuracy for quantitative imaging across a large field of view10, it requires complex alignment, ultimately limiting its integration across various imaging modalities, and reducing their flexibility. Additionally, there are much sophisticated single element uniform illuminators, such as Top Shape (Asphericon Inc.)11 and Moiseev et al.40, for quantitative fluorescence imaging. As with other solutions, this is a costly optical design that cannot be adapted for a diverse array of widefield modalities. Furthermore, combining multiple color sources within a single uniform illumination setup presents additional challenges which further limits their application in widefield microscopy.
Recently, Çelebi and Aslan et al. (2023) developed a uniform diffuse illuminator that adapted the concept of an integrating sphere, but in the shape of a cone, to maximize coupling efficiency and field uniformity while maintaining simplicity and low cost in their design14. Building on the work of Çelebi and Aslan et al. (2023), we have updated the Effective Uniform Color-Light Integration Device (EUCLID) illumination system, and here we present and qualitatively and quantitatively assess the implementation of a simplified EUCLID design for imaging in birefringence microscopy (BRM) and for the interferometric reflectance imaging sensor (IRIS), to demonstrate the ease of use for an array of widefield imaging schemes, especially in whole slide imaging (WSI). This work focuses on the application of EUCLID as a robust illumination system designed to enhance uniformity, thereby improving image quality for visualization as well as the data fidelity of quantitative imaging. First, we show that widefield mosaic stitching using EUCLID achieves comparable results to traditional Köhler illumination coupled with post-processing computational correction, but without the need for computational correction and a significant improvement in overall SNR. Next, to demonstrate the benefits of EUCLID for quantitative microscopy, we highlight the sensitivity of quantitative birefringence microscopy to illumination uniformity and demonstrate that EUCLID enhances quantitative analysis of tissue images. In the last section of this paper, we analyzed quantitative performance of EUCLID in a scanning epi-illumination setup and compared the results to that of IRIS measurement with a commercial illuminator. While we utilized EUCLID to demonstrate the importance of illumination uniformity for quantitative and qualitative birefringence microscopy and IRIS, the benefits are more general, as the design can be easily adapted to other widefield microscopy and mesoscopic imaging modalities.
Results and discussion
Low-cost EUCLID for widefield microscopy
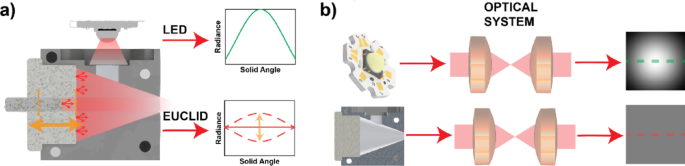
The primary criterion for a uniform irradiance profile with the EUCLID design is the use of a conical surface and a broad-band diffuse reflectance material with Lambertian angular reflectance (e.g. titanium dioxide or barium sulfate-based paints, or polymer-based diffuse reflecting suspensions such as Spectralon [Labsphere Inc.], etc.) (Fig. 1a). The EUCLID device corrects the unequal radiance profile of traditional LEDs to create a uniform radiance profile on the sample plane over a large field of view (Fig. 1a,b). Similar to an integrating sphere, light entering the EUCLID undergoes many diffusive reflections, which homogenize any spatial structure of the input light, effectively creating many point sources inside the cavity. Because of its conical structure, any output light originates from the back surface of the conical cavity, optimizing optical coupling and uniformity14. The EUCLID structure has a plunger on its back surface to control its output radiance. The plunger allows EUCLID the flexibility to adjust the resulting illumination profile for any illumination setup (Fig. 1a). The original EUCLID design was fabricated from high density polytetrofluoroethylene (PTFE), which made it costly, challenging to machine precisely, and mechanically limited for attaching to external optical components14. To simplify the manufacturing process, and to improve the mechanical compatibility with optical systems, the new EUCLID device has been machined of aluminum, and the internal surfaces have been coated with a highly diffusive paint (Avian-B White Reflectance Coating – Avian Technologies) to decrease cost and overall complexity. The paint provides a reflectance of > 97% over the range of 350–850 nm41. A block of aluminum is CNC machined in two separate pieces that snap together to form a tight optical seal (Figure S1). The inner surfaces of the EUCLID device are sandblasted, to enhance adhesion of the paint to the aluminum surface; and an airbrush is used to apply ~ 20 coats of the diffusely reflecting paint, for a total paint thickness of ~ 1 mm, to achieve broadband Lambertian diffuse reflectance. After coating, the pieces assemble using screws to secure them to a backplate, a process made more straightforward by the machinability of aluminum. In addition to simplifying assembly, the use of aluminum allows for more efficient heat dissipation with higher power LEDs. The aluminum structure can be easily integrated with heat sinks, which passively dissipate heat generated by the light source, ensuring stable operation during extended imaging sessions, which is important for maintaining consistent illumination over time. The level of uniformity achieved is comparable to our previous design, as demonstrated in14, with < 2% variation across the imaging plane, benefiting applications of widefield imaging and tiled imaging.
Schematic illustrating the principle of EUCLID illumination compared to conventional LED-based illumination systems. (a) Theoretical radiance profiles of a convention LED vs. and LED coupled to the EUCLID device. The dashed lines illustrate the adjustable radiance profile of EUCLID with respect to the rod’s position. (b) Demonstrations of the illumination profiles of two sources (LED vs. LED coupled to EUCLID) after passing through a system traditional microscope system setup for Köhler illumination. A full cross-sectional diagram of the EUCLID device can be found in supplementary Figure S1.
High-throughput widefield imaging with EUCLID
Non-uniform illumination degrades image quality for widefield stitching due to shading and vignetting’s artifacts. These effects can be mitigated by acquiring images with a larger overlap of tiles; however, this requires a larger number of images to cover the same field of view, whereas uniform illumination enables higher throughput imaging as less overlap is required for proper stitching across a larger tiled FOV11. Depending on the objective FOV used for imaging, the optimal tile overlap can be adjusted to ensure seamless stitching while balancing imaging throughput. Achieving uniform illumination traditionally requires precise alignment of illumination optics, which can be labor-intensive and setup-dependent. EUCLID, on the other hand, provides an adjustable illumination field profile by adjusting the reflective rod (Fig. 1a), allowing for easy customization of field uniformity across different objectives. This adaptability makes EUCLID a universal solution for uniform illumination in microscopy, reducing the need for meticulous optical alignment and enabling more consistent widefield imaging across diverse platforms.
To evaluate the effect of non-uniform illumination on tile stitching in microscopy, and to assess the efficacy of EUCLID illumination, CCP-BRM tiled image sets were acquired at two magnifications, 4 × and 10x, to compare imaging with three illumination conditions: (1) traditional Köhler illumination (Fig. 2a,d); (2) Köhler illumination with BaSiC correction (Fig. 2b,e); and (3) a EUCLID illumination setup (Fig. 2c,f). In this implementation, CCP-BRM enables imaging of myelin structure in thin brain slices and is used to evaluate structural changes in various models for neurodegenerative diseases32,33. At 4X magnification (NA: 0.13), a 12 × 9 grid of images of a 30-micron-thick rhesus monkey coronal brain section was acquired with 10% overlap using CCP-BRM (see “Transmission imaging with birefringence microscopy” in the Methods section). Shading and vignetting artifacts are evident under traditional Köhler illumination (Fig. 2a) but are effectively eliminated with post processing BaSiC correction (Fig. 2b). EUCLID illumination, on the other hand, does not require post processing correction, rendering a seamless image across the entire field of view comparable to the stitched image with BaSiC correction (Fig. 2c). Although BaSiC correction effectively improves uniformity for stitching in most cases, it cannot correct for the effective loss of dynamic range caused by the nonuniformities. For example, if 30% of the light is lost at the edges due to vignetting, there is an equivalent 30% reduction in effective dynamic range in those regions. This becomes increasingly important in the case of high contrast samples (containing both bright and dim objects) where BaSiC correction breaks down for pixels that do not provide sufficient signal or become saturated.
Stitching artifacts for qualitative widefield image stitching with CCP-BRM. Images acquired at (a–c) 4X (NA: 0.13) and (d–f) 10X (NA: 0.3) of a coronal rhesus monkey brain section under different illumination conditions: (a, d) Köhler CCP-BRM, (b, e) CCP-BRM + BaSIC, and (c, f) EUCLID CCP-BRM. Scale = 5 mm.
At 10X magnification (NA: 0.3), the minimum resolution needed to visualize individual myelinated fibers, the impact of stitching artifacts is less pronounced at 10% overlap, but still apparent at 1% overlap (Fig. 2d). However, even at this minimal overlap, EUCLID illumination provides seamless stitching (Fig. 2f) similar to BaSiC correction (Fig. 2e). At this resolution, the increased number of images required to cover the same FOV significantly raises the computational demands (computational time and memory requirements) of BaSiC correction, reducing its practicality for applications with limited computational resources for high throughput data acquisition. A significantly larger number of images required to cover the same field of view (FOV) substantially increases the computational time and memory demands of BaSiC correction, making it impractical for high-throughput applications with limited computational resources. With 1% overlap, BaSiC correction for a half-hemisphere tissue section (~ 600 images, 9MP @ 16-bit depth; ~ 10 GB total) requires approximately 12 min to load the data to memory, followed by an additional 4–6 min for correction processing. For larger tissue sections (human brain), these demands scale further, leading to excessive processing times and memory requirements. An alternative approach to post processing, to reduce the stitching artifacts, is the acquisition of the tiled image set with a larger overlap (> 10%). At this resolution, for large samples, this has an impact on the acquisition time which would require a significant increase in the total number of images acquired across the entire tissue sample. At this resolution, imaging a single hemisphere requires ~ 600 images at 1% overlap. Increasing to 10% overlap results in an approximately 20% increase in the total number of images needed, leading to an equivalent 20% increase in acquisition time. This effect is particularly critical when imaging large brain tissue sections, such as full human brain samples, which may require 2500–5000 images at 1% overlap. In such cases, the 20% increase in the number of images from higher overlap settings results in significantly longer acquisition. This demonstrates that EUCLID illumination offers a robust approach for widefield imaging at multiple scales for higher throughput imaging and seamless stitching with minimal overlap, which is essential for efficiently capturing large and complex tissue samples.
Uniform Illumination improves accuracy of quantitative birefringence microscopy (qBRM)
Widefield quantitative imaging techniques are crucial for analyzing structural and functional properties of biological specimen but are often negatively impacted by non-uniform illumination. In many widefield techniques, image intensity variations are used to extract quantitative information about biological samples using a wide array of computational and imaging techniques. Especially in brightfield imaging, where light is abundant and shot noise is limited by the characteristics of imaging sensor, an unequal illumination distribution across the imaging FOV causes an unequal noise distribution (shot noise dominated) which results in errors in downstream quantitative analysis. This is particularly important for low contrast (or high dynamic range) measurements with lower SNR. This variability introduces errors in methods ranging from quantitative fluorescence microscopy4,11,42, quantitative phase imaging24, and polarized light imaging methods29,43. qBRM (see “Transmission imaging with birefringence microscopy” in the Methods section) is exemplary of these widefield quantitative imaging techniques, which relies on intensity information to computationally solve for quantitative parameter maps of relative retardance and optic-axis orientation of thin biological specimen. This process makes qBRM highly sensitive to noise, particularly shot noise, and illumination non-uniformity, as any irregularities in intensity directly affect the accuracy of the extracted birefringence parameters. Computational correction methods, such as BaSiC and CIDRE, mitigate illumination non-uniformity. However, they are not suitable for quantitative imaging techniques like qBRM and IRIS. Specifically, the response of polarization signals is inherently nonlinear, making illumination compensation difficult with methods like BaSiC10.
To quantitatively assess the effect of non-uniform illumination on qBRM, we conducted simulations to examine the effect of shot noise and illumination uniformity on the accuracy of extracted birefringence parameters (Figure S3). We examined the probability density functions (PDF) of the relative retardance and optic-axis orientation distributions under Köhler (Gaussian intensity profile) and EUCLID (uniform intensity profile) illumination both in real sample and simulations with added shot noise (modeled as (sqrt{N}), where N is the simulated photoelectron count). The simulations were used to investigate changes in extraction accuracy of samples with varying birefringence levels (low and high retardance) as well as under different imaging conditions (low and high well depth imaging). These simulations reveal that non-uniform illumination under Köhler conditions significantly widens the distribution of extracted values for both relative retardance and optic-axis orientation, when compared with the uniform illumination scheme (EUCLID) (Table S1). These effects are especially significant in areas of weak birefringence (low retardance), where shot noise dominates, as the signal level is low, affecting the extracted values. This leads to a large broadening of the PDF for both relative retardance in the Köhler illumination case. In contrast, areas of strong birefringence signal, the effect is less apparent but there is a broadening of the distribution in the Köhler illumination cases. Additionally, the simulations demonstrated that the effect of illumination non-uniformity is exacerbated in low well-depth imaging scenarios. Limited well-depth increases noise contributions, further degrading the effective dynamic range and accuracy of the extracted parameters. To validate the simulation results, we imaged a 30-micron thick coronal brain section from a rhesus monkey specimen with qBRM. A 4X tile scan of the entire brain section (Fig. 3a) producing a 12 × 9 grid, was acquired with 10% overlap under both Köhler and EUCLID illumination conditions. qBRM images are rendered with a custom color wheel (Fig. 3a, top right), where the color corresponds to the optic-axis orientation and the brightness of each pixel corresponds to the relative retardance32. Manually selected regions of interest (ROIs) in white (strong birefringence) and gray matter (weak birefringence) were analyzed to compare the PDFs of retardance and optic-axis orientation within these regions.
Impact of illumination uniformity on qBRM analysis of relative retardance and optic-axis orientation. 4X qBRM tile scan of the entire brain section (a), 12 × 9 grid, was acquired with 10% overlap under both Köhler and EUCLID illumination conditions in (b, c) gray matter and (d, e) white matter regions. The effect of the illumination scheme in the extraction of relative retardance and optic-axis orientation is evident in gray matter, where there is a clear broadening the of the peaks for both values. This effect is less pronounced for white matter, but the orientation distribution has more pronounced peaks under EUCLID illumination showing three distinct fiber tracts (red arrows).
In gray matter (Fig. 3b,c), the PDF under Köhler illumination show a broader distribution for both retardance and optic-axis orientation, indicating increased uncertainty caused by non-uniform illumination. The peak value of the relative retardance distribution increased by 43.3% indicating an improved sensitivity under uniform illumination. Additionally, there is a noticeable shift in the mean extracted retardance value under Köhler illumination. The shift is attributed to shot noise, as the retardance values are bounded by zero, where noise causes the distribution to skew towards higher values. Similarly, the distribution of the optic-axis orientation from this ROI has a peak value increase of 25%. This effect is less pronounced for orientation in gray matter as there are no large fiber tracts present within the ROI, leading to a more uniform distribution of orientation. In white matter (Fig. 3d,e), the difference observed between the two illumination schemes is less pronounced, as the stronger birefringence signal in white matter reduces the influence of shot noise. However, Köhler illumination still exhibits a slight broadening of the retardance compared to EUCLID illumination with a peak value increase of 8% under uniform illumination. The optic-axis orientation PDF, on the other hand, demonstrates that under uniform illumination there is a clear increase in the contrast between peaks of three distinct nerve-fiber tracks (Fig. 3a, red arrows) when compared to the traditional Köhler illumination. The peak values for each of these tracts had increases of 20.5%, 9.4%, and 15.7% for the three peaks respectively under EUCLID illumination. These results underscore the importance of uniform illumination in the accuracy of extracting the birefringence parameters, which has implications for various neuroscience applications28,29,31,33,44. Moreover, a uniform light distribution also helps to use the full dynamic range of the imaging sensor much more efficiently. A narrower distribution in high-contrast readings can allow for an increased exposure time, optimizing the shot-noise of the low-contrast regions (Figure S6). These results demonstrate that EUCLID illumination significantly improves the quantitative analysis of qBRM by enhancing the accuracy of orientation and relative retardance measurements, a benefit that extends to other quantitative imaging techniques, although the degree of improvement may vary depending on the specific imaging method.
Spatial light distribution impact on reflectance microscopy with IRIS
IRIS is a wide-field imaging technique designed to quantify molecular interactions on its sensor by detecting subtle regional reflectance changes which are due to ensemble response of the small particles of interest. It also utilizes multiple wavelengths to monitor environmental effects, such as bulk-effect45, resulting in sensitive and accurate molecular binding results. Due to its interferometric nature, the signal, reflectance changes, can be read as intensity with a CMOS camera. Thus, an even intensity distribution over the entire area of its sensor, typically 5 × 7 mm, is crucial for accurate and highly multiplexed IRIS measurements, but these issues are not unique to IRIS. For instance, the relative spatial distribution of the selected wavelength channels must be uniform for spectral pixel-diversity IRIS, which detects single viruses by comparing their optical responses across the employed wavelengths35. In epifluorescence microscopes, the emitted light which constitutes the signal purely depends on the excitation light. Any unevenness in the spatial distribution of the excitation could cause false signals. Characterization of cellular activities also benefits from a compact, even and powerful excitation light39,46,47, which can minimize animal stress during in-vivo experiments. Moreover, such light sources would increase the experimental throughput of optogenetics studies as demonstrated in vitro screening in ubiquitous well plates where spatiotemporal control of illumination is important to control cellular activities in each well23.
The signal in IRIS is built on the interference of the reflected fields, typically from an SiO2 grown Si chip. The chip is illuminated with partially coherent light at normal incidence, and the reflected light is collected onto a CMOS imaging sensor, where the interference occurs and is read as light intensity. In the ensemble biosensing modality of IRIS, this transduction is analyzed for multiple spectra of light at different regions in the field-of-view of the imaging sensor. This is key for determining biomass accumulation without any matrix effects48,49,50. In the single particle modality of IRIS, the spectral intensity variations at the same location are compared to detect the presence of particles. The signal relies on local variations of different spectral light35. Therefore, the global and local distribution of illumination light is crucial for accurate characterization of biomass accumulation and particle detection (Figure S4).
A light source that uniformly provides multiple spectral outputs from a single small output port is essential for advanced IRIS technologies. A conventional method for multi-spectral illumination involves coupling multi-wavelength light into a multi-mode light guide using various combinations of dichroic elements. A multi-mode light guide can deliver high power and uniform light from its output aperture due to multiple random total internal reflection events. However, the light distribution for multi-spectral illumination varies for each spectral component also due to non-uniform mixing inside the guide14. In the EUCLID system, light is uniformly mixed inside the cavity, as studied in our previous publication14. In this section, we assess and compare the performance of EUCLID, and an off-the-shelf liquid light guide (LLG)-coupled light source (Chrolis, Thorlabs) in IRIS applications.
A wide field of view is crucial for many imaging techniques to achieve highly multiplexed results. In IRIS, it is essential for screening biomass accumulation on multiple spots. We built a scanning IRIS setup that can screen a 5 × 7 mm area with a 4 × objective lens. To cover this wide region, six different image tiles were captured and stitched together to create the final wide-field, high-resolution images. The spotted biomasses (single-stranded DNA molecules) on the IRIS chip are expected to have the same optical thickness. We analyzed the same chip using two different illumination sources: (i) an LLG-coupled light source and (ii) EUCLID.
First, we qualitatively assessed the local and global light distribution by tracking changes in the histogram shape for multiple FOVs (Figure S5, S6). EUCLID provides a uniform and globally consistent illumination distribution, where three distinct peaks for the oxide background, silicon reference, and biomass spot are visible (Figure S5). In the presence of non-uniform illumination, the peak of the biomass spot is only distinguishable for a small portion of the field of view, merging into the oxide background as the field of view expands due to stitching artifacts and non-uniform distribution within one tile (Fig. 4a,b).
Stitched images of 7 × 5 mm a dry IRIS chip illuminated under a LLG coupled device (CHROLIS, Thorlabs) (a) and EUCLID (b), where the distance between two vertical rectangle is 200 μm. The contrasts are adjusted to be the same. The histograms of those stitched images are given in (b) and (e). The peaks of the histograms are labelled with red stars which corresponds to oxide backgrounds, spots and silicon references. In image (a), the spot and background histogram are merged (b), thus, it has two separate peaks. Under EUCLID illumination, each region has 1.56%, 1.89% and 1.61% plateau uniformity, respectively. The thickness histograms are calculated for a wet chip and given in (c) and (f) for (a) and (d) illumination. The calculated mean and standard deviation for fitted gaussians are 2.40 nm and 1.04 nm for (c) and 1.86 nm and 0.65 nm for (f).
We then quantified the histogram metrics of the entire stitched image for EUCLID illumination. EUCLID provides illumination with 1.56%, 1.89%, and 1.61% plateau uniformity for the oxide background, spot, and silicon reference, respectively (Fig. 4d,e). Following this analysis, we quantified the biomass in terms of optical thickness50. The calculated thicknesses are shown in Fig. 4c,f, and a Gaussian is fitted to each histogram to determine thickness accuracy. Since the thickness values of the spots are expected to be similar, narrower deviation in the fitted Gaussian indicates more accurate thickness calculation. Consequently, the deviations are found to be 0.65 nm and 1.04 nm for EUCLID and conventional illumination, respectively.
Conclusion
In this study, we demonstrate that uniform illumination is critical for quantitative and qualitative widefield imaging with two exemplary widefield imaging techniques, birefringence microscopy (BRM) and interferometric imaging sensor (IRIS) with a field of view of 3.15 × 3.15 mm and 5 × 7 mm, respectively. We describe an updated design for the effective uniform color light integration device (EUCLID) which is potentially a universal and simple-to-implement solution for many widefield imaging applications. Using BRM and IRIS as exemplary techniques, we demonstrate that the use of EUCLID significantly improves image stitching for large-scale qualitative imaging, increases the throughput of the entire process and enhances measurement accuracy in quantitative analyses with both techniques. The examples provided pertain to high-light applications, where the power is constrained by the characteristics of the imaging sensor. We have demonstrated the ability of EUCLID to enhance the visibility of low-contrast signals through improved uniformity. Although the performance in low-light applications is yet to be demonstrated experimentally, the ability to enhance uniformity and efficiency of illumination is expected to have a positive impact in applications such as fluorescence microscopy. For dark-field illumination, a detailed efficiency study is also required. Based on our previous findings, we strongly believe that EUCLID can deliver superior uniformity while maintaining efficient power delivery in broad range of optical systems. We also demonstrated that when EUCLID is coupled with multimode light guides, it exhibits enhanced light mixing properties. This capability could be valuable for generating multi-colored illumination from a multi-fiber system or achieving speckle-free laser illumination51(see Figure S7, S8).
This work focused on the application of EUCLID to the above techniques, however, the design of EUCLID can be easily adopted by several widefield imaging techniques including (but not limited to) quantitative fluorescence, quantitative phase contrast imaging, polarized light imaging, and optogenetics. Future work will look to explore the various imaging applications where EUCLID can enhance imaging and quantitative analysis.
Methods
Imaging setups
Transmission imaging with birefringence microscopy
Birefringence microscopy (BRM) is a label-free imaging technique that utilizes changes in light polarization induced by birefringent structures in the sample to generate contrast. Here we use BRM as a representative technique to demonstrate the impact of illumination uniformity on widefield imaging for both qualitative and quantitative applications. In our implementation, BRM combines two complementary imaging techniques: qualitative imaging with crossed circular polarizers (CCP-BRM), and quantitative birefringence imaging (qBRM)31,32,33 (See Figure S2a). In brief, CCP-BRM is a qualitative imaging technique that enables real-time structural imaging of birefringent specimen by imaging through a pair of crossed circular polarizers52. For quantitative imaging, qBRM, a series of images are acquired at various linear polarization angles of illumination, coupled with a circular polarization analyzer at the camera, and intensity variations under different illumination conditions can be modeled with Jones calculus to extract parameter maps of relative retardance (density of birefringence) and optic-axis orientation of birefringent materials.
As described in32, our custom-built birefringence microscope (BRM) system is based on a dual-retarder setup with a polarization state generator (PSG) and analyzer (PSA) on either side of the sample (Figure S2a). The system uses a high-power LED (Thorlabs M625L4, 625 nm), which is collimated one of two ways: (1) coupled with the EUCLID device (aperture diameter 4 mm) for uniform illumination and collimated with a planoconvex lens, or (2) more conventionally, collimated by an aspheric condenser lens with a diffusor on the plano surface (Thorlabs ACL2520U-DG6-B) to form Köhler illumination. The aspheric condenser lens with a diffusor surface is a commercially available solution specifically designed to enhance uniformity in LED-based illumination setups. Collimated light passes through polarizing and waveplate components to control the polarization state of the illumination and is then sent through a condenser lens for the traditional Köhler illumination scheme. Similar components serve as polarization analyzer after the sample. Thin-film polarizers and true zero-order quarter-wave plates (Thorlabs WPQ10ME-633) enable precise polarization control, supporting both circularly polarized CCP-BRM and qBRM imaging modes on either side of the sample. Light is passed through the sample and the detection side polarization optics and collected by strain free objectives, to minimize residual birefringence, and images are captured with a high-speed, cooled camera (Teledyne Iris 9, 9MP @ 16-bit, 30 FPS). A Fast XY scanning stage (Thorlabs MLS203-1) and a Z-scanning stage (Thorlabs ZFM2020) are used for acquiring tiled image sets across the sample. In our studies, the image tiles were acquired with a variable overlap and stitched using the FIJI plugin with linear blending53.
Reflectance imaging with IRIS
The IRIS system is a mesoscopic-scale interferometric imaging system that measures changes in sample thickness with sub-nanometer resolution, over many spots on a Si-SiO2 chip. The reflectance setup is configured to provide normal-incidence illumination to the screened sample (Figure S2b). Two identical collimating lenses image the output of the light source on the back focal plane of the objective lens (Nikon 4x, 0.1 NA). The illumination partially fills the back focal plane to provide small-angle illumination to the sample. The reflected light from the sample is collected with the same objective lens providing the illumination and the collected light is imaged on to a CMOS sensor using a 200 mm tube lens. For the light source, two different devices have been tested and evaluated: (1) A RGBY LED is connected to EUCLID (aperture diameter 4 mm), providing illumination at 635, 530, 460 and 560 nm, respectively, and (2) a standard multicolor illuminator (Thorlabs CHROLIS), which has five different LEDs. The sample consists of a silicon chip with 110 nm thermally grown oxide top layer. Using conventional photolithography, the chip is patterned where the oxide layer is etched down to Si surface. The circular rings (Fig. 4a,d) indicate where the capture probes (in this particular case DNA molecules) are immobilized.
The sample is inserted into a custom chip holder and the custom holder is mounted onto a 3-axis Nanomax stage. Focus adjustments and XY scanning were performed by three stepper motor actuators. The acquired tiles were stitched using the same FIJI plugin as described above (see Sect. 2.2.1).
Brain section preparation for BRM
Monkeys were acquired from World Wide Primates, Inc. or National Primate Research Centers, and were maintained in the Animal Science Center of Boston University Medical Campus (fully AAALAC accredited). All procedures were approved by the Boston University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and other relevant institutional and federal regulations. The authors affirm compliance with the ARRIVE guidelines for reporting animal research. During the study, monkeys were individually housed in a colony room, and enrichment procedures that met or exceeded USDA requirements were offered.
Samples for birefringence imaging were prepared according the protocol described in32. In brief, brain tissue from a rhesus monkey brain was perfusion fixed with 37 °C 4% paraformaldehyde and placed into the same solution overnight. 30-micron thick frozen sections are cut from each slab and mounted onto a microscope slide with 85% glycerol (15% deionized water) for index matching to reduce optical scattering. This ensures optimal imaging conditions and enhances the visibility of the contrast mechanism of interest (birefringence), yielding optimal images of birefringent structures.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
-
Gupta, E., Bhalla, P., Khurana, N. & Singh, T. Histopathology for the diagnosis of infectious diseases. Indian J. Med. Microbiol. 27(2), 100–106 (2009).
-
Chen, Z. et al. Histological quantitation of brain injury using whole slide imaging: A pilot validation study in mice. PLoS ONE 9(3), e92133 (2014).
-
Valdes, P. A., Juvekar, P., Agar, N. Y. R., Gioux, S. & Golby, A. J. Quantitative wide-field imaging techniques for fluorescence guided neurosurgery. Front. Surg. 6, 31 (2019).
-
Buntz, A. et al. Quantitative fluorescence imaging determines the absolute number of locked nucleic acid oligonucleotides needed for suppression of target gene expression. Nucleic Acids Res. 47(2), 953–969. https://doi.org/10.1093/nar/gky1158 (2019).
-
Lu, X. et al. Widefield imaging of rapid pan-cortical voltage dynamics with an indicator evolved for one-photon microscopy. Nat. Commun. 14(1), 6423. https://doi.org/10.1038/s41467-023-41975-3 (2023).
-
Cramer, J. V. et al. In vivo widefield calcium imaging of the mouse cortex for analysis of network connectivity in health and brain disease. Neuroimage 199, 570–584. https://doi.org/10.1016/j.neuroimage.2019.06.014 (2019).
-
Ji, Y., Zeng, C., Tan, F., Feng, A. & Han, J. Non-uniformity correction of wide field of view imaging system. Opt. Express 30(12), 22123–22134. https://doi.org/10.1364/OE.458180 (2022).
-
Rimmer, M. P. Relative illumination calculations. In Proc.SPIE; Vol. 0655, pp 99–104 (1986). https://doi.org/10.1117/12.938414.
-
Dross, O., Mohedano, R., Hernández, M., Cvetković, A., Miñano, J. C., Benítez, P. Köhler integrators embedded into illumination optics add functionality. In Optical systems design; (2008).
-
Guo, W., Song, J., Zeng, N. & Ma, H. Mueller matrix imaging optimized by uniform illumination. Front. Phys. 10, 931958. https://doi.org/10.3389/fphy.2022.931958 (2022).
-
Khaw, I. et al. Flat-field illumination for quantitative fluorescence imaging. Opt. Express 26(12), 15276–15288. https://doi.org/10.1364/OE.26.015276 (2018).
-
Sawyer, T. W., Luthman, A. S. & Bohndiek, S. E. Evaluation of illumination system uniformity for wide-field biomedical hyperspectral imaging. J. Opt. (UK) 19(4), 045301. https://doi.org/10.1088/2040-8986/aa6176 (2017).
-
Mau, A., Friedl, K., Leterrier, C., Bourg, N. & Lévêque-Fort, S. Fast Widefield scan provides tunable and uniform illumination optimizing super-resolution microscopy on large fields. Nat. Commun. 12(1), 3077. https://doi.org/10.1038/s41467-021-23405-4 (2021).
-
Çelebi, İ, Aslan, M. & Ünlü, M. S. A spatially uniform illumination source for widefield multi-spectral optical microscopy. PLoS ONE 18(10), e0286988. https://doi.org/10.1371/journal.pone.0286988 (2023).
-
Smith, K. et al. CIDRE: An illumination-correction method for optical microscopy. Nat. Methods 12(5), 404–406. https://doi.org/10.1038/nmeth.3323 (2015).
-
Peng, T. et al. A BaSiC tool for background and shading correction of optical microscopy images. Nat. Commun. 8(1), 14836. https://doi.org/10.1038/ncomms14836 (2017).
-
Gaborski, T. R., Sealander, M. N., Ehrenberg, M., Waugh, R. E. & McGrath, J. L. Image correlation microscopy for uniform illumination. J. Microsc. 237(1), 39–50. https://doi.org/10.1111/j.1365-2818.2009.03300.x (2010).
-
Piccinini, F. & Bevilacqua, A. Colour vignetting correction for microscopy image mosaics used for quantitative analyses. Biomed. Res. Int. 2018, 7082154. https://doi.org/10.1155/2018/7082154 (2018).
-
Syed, T. Q., Vigneron, V., Lelandais, S., Barlovatz-Meimon, G., Malo, M., Charriere-Bertrand, C., Montagne, C. Detection and counting of “in Vivo” cells to predict cell migratory potential. In 2008 first workshops on image processing theory, tools and applications; pp 1–8 (2008). https://doi.org/10.1109/IPTA.2008.4743748.
-
Dimopoulos, S., Mayer, C. E., Rudolf, F. & Stelling, J. Accurate cell segmentation in microscopy images using membrane patterns. Bioinformatics 30(18), 2644–2651. https://doi.org/10.1093/bioinformatics/btu302 (2014).
-
Chalfoun, J. et al. MIST: Accurate and scalable microscopy image stitching tool with stage modeling and error minimization. Sci. Rep. 7(1), 4988. https://doi.org/10.1038/s41598-017-04567-y (2017).
-
Xu, F. et al. High-throughput mapping of a whole rhesus monkey brain at micrometer resolution. Nat. Biotechnol. 39(12), 1521–1528. https://doi.org/10.1038/s41587-021-00986-5 (2021).
-
Repina, N. A. et al. Engineered illumination devices for optogenetic control of cellular signaling dynamics. Cell Rep. 31(10), 107737. https://doi.org/10.1016/j.celrep.2020.107737 (2020).
-
Curl, C. L. et al. Quantitative phase microscopy: A new tool for measurement of cell culture growth and confluency in situ. Pflugers. Arch. 448(4), 462–468. https://doi.org/10.1007/s00424-004-1248-7 (2004).
-
Wang, Z. et al. Spatial light interference microscopy (SLIM). Opt. Express 19(2), 1016–1026. https://doi.org/10.1364/OE.19.001016 (2011).
-
Chen, X., Kandel, M. E. & Popescu, G. Spatial light interference microscopy: Principle and applications to biomedicine. Adv. Opt. Photonics 13(2), 353. https://doi.org/10.1364/aop.417837 (2021).
-
Morrison, L. E. et al. Brightfield multiplex immunohistochemistry with multispectral imaging. Lab. Invest. 100(8), 1124–1136. https://doi.org/10.1038/s41374-020-0429-0 (2020).
-
Axer, M. et al. A novel approach to the human connectome: Ultra-high resolution mapping of fiber tracts in the brain. Neuroimage 54(2), 1091–1101. https://doi.org/10.1016/j.neuroimage.2010.08.075 (2011).
-
Axer, M. et al. High-resolution fiber tract reconstruction in the human brain by means of three-dimensional polarized light imaging. Front. Neuroinform. 5, 34. https://doi.org/10.3389/fninf.2011.00034 (2011).
-
Menzel, M. et al. Automated computation of nerve fibre inclinations from 3d polarised light imaging measurements of brain tissue. Sci. Rep. 12(1), 4328. https://doi.org/10.1038/s41598-022-08140-0 (2022).
-
Morgan, M. L., Brideau, C., Teo, W., Caprariello, A. V. & Stys, P. K. Label-free assessment of myelin status using birefringence microscopy. J. Neurosci. Methods 360, 109226. https://doi.org/10.1016/j.jneumeth.2021.109226 (2021).
-
Blanke, N. et al. Practical considerations for birefringence microscopy of myelin structure: Microscope design and tissue processing for effective imaging. Imaging Neurosci. 2, 1–22. https://doi.org/10.1162/imag_a_00186 (2024).
-
Blanke, N., Go, V., Rosene, D. L. & Bigio, I. J. Quantitative birefringence microscopy for imaging the structural integrity of CNS myelin following circumscribed cortical injury in the rhesus monkey. Neurophotonics 8(01), 015010. https://doi.org/10.1117/1.nph.8.1.015010 (2021).
-
Park, J. S., Soh, J. W. & Cho, N. I. Generation of high dynamic range illumination from a single image for the enhancement of undesirably illuminated images. Multimed. Tools Appl. 78(14), 20263–20283. https://doi.org/10.1007/s11042-019-7384-z (2019).
-
Aslan, M. et al. A label-free optical biosensor-based point-of-care test for the rapid detection of monkeypox virus. Biosens. Bioelectron. 269, 116932. https://doi.org/10.1016/j.bios.2024.116932 (2025).
-
Coumans, F. A. W., van der Pol, E. & Terstappen, L. W. M. M. Flat-top illumination profile in an epifluorescence microscope by dual microlens arrays. Cytometry A 81A(4), 324–331. https://doi.org/10.1002/cyto.a.22029 (2012).
-
Ryu, J. et al. High-Speed time-resolved laser-scanning microscopy using the line-to-pixel referencing method. Appl. Opt. 55(32), 9033–9041. https://doi.org/10.1364/AO.55.009033 (2016).
-
Ibrahim, K. A., Mahecic, D. & Manley, S. Characterization of flat-fielding systems for quantitative microscopy. Opt. Express 28(15), 22036–22048. https://doi.org/10.1364/OE.395900 (2020).
-
Douglass, K. M., Sieben, C., Archetti, A., Lambert, A. & Manley, S. Super-resolution imaging of multiple cells by optimized flat-field epi-illumination. Nat. Photon. 10(11), 705–708. https://doi.org/10.1038/nphoton.2016.200 (2016).
-
Moiseev, M. A., Byzov, E. V., Kravchenko, S. V. & Doskolovich, L. L. Design of LED refractive optics with predetermined balance of ray deflection angles between inner and outer surfaces. Opt. Express 23(19), A1140–A1148. https://doi.org/10.1364/OE.23.0A1140 (2015).
-
Avian Technologies LLC. Avian-B White Reflectance Coating. https://aviantechnologies.com/product/avian-b-white-reflectance-coating/ (accessed 2024–12–19).
-
Waters, J. Accuracy and precision in quantitative fluorescence microscopy. J. Cell Biol. 185, 1135–1148. https://doi.org/10.1083/jcb.200903097 (2009).
-
Yang, B. et al. Instant polarized light microscopy for imaging collagen microarchitecture and dynamics. J. Biophotonics 14(2), e202000326. https://doi.org/10.1002/jbio.202000326 (2021).
-
Bosticardo, S. et al. Evaluation of tractography-based myelin-weighted connectivity across the lifespan. Front. Neurosci. 17, 1228952. https://doi.org/10.3389/fnins.2023.1228952 (2023).
-
Marn, A. M., Chiodi, E. & Ünlü, M. S. Bulk-effect-free method for binding kinetic measurements enabling small-molecule affinity characterization. ACS Omega 6(10), 6836–6841. https://doi.org/10.1021/acsomega.0c05994 (2021).
-
Milstein, J. N., Nino, D. F., Zhou, X. & Gradinaru, C. C. Single-molecule counting applied to the study of GPCR oligomerization. Biophys. J. 121(17), 3175–3187. https://doi.org/10.1016/j.bpj.2022.07.034 (2022).
-
Doran, P. R. et al. Widefield in vivo imaging system with two fluorescence and two reflectance channels, a single SCMOS detector, and shielded illumination. Neurophotonics 11(3), 034310. https://doi.org/10.1117/1.NPh.11.3.034310 (2024).
-
Vedula, R. et al. Self-referencing substrates for optical interferometric biosensors. J. Mod. Opt. 57(16), 1564–1569. https://doi.org/10.1080/09500340.2010.507883 (2010).
-
Daaboul, G. G. et al. LED-based interferometric reflectance imaging sensor for quantitative dynamic monitoring of biomolecular interactions. Biosens. Bioelectron. 26(5), 2221–2227. https://doi.org/10.1016/j.bios.2010.09.038 (2011).
-
Avci, O., Ünlü, N. L., Özkumur, A. Y. & Ünlü, M. S. Interferometric reflectance imaging sensor (IRIS)—A platform technology for multiplexed diagnostics and digital detection. Sensors (Switzerland) 15, 17649–17665. https://doi.org/10.3390/s150717649 (2015).
-
Ünlü, M. S., Aslan, M., Celebi, I., Yurdakul, C., Marn, A. M. Efficient and uniform color-light integration device. US12140775B2, November 12, 2024. https://patents.google.com/patent/US12140775B2/en (accessed 2025–05–29).
-
Glazer, A. M., Lewis, J. G. & Kaminsky, W. An automatic optical imaging system for birefringent media. Proc. Royal Soc. London Ser. A Math. Phys. Eng. Sci. 1996(452), 2751–2765. https://doi.org/10.1098/rspa.1996.0145 (1955).
-
Preibisch, S., Saalfeld, S. & Tomancak, P. Globally optimal stitching of tiled 3d microscopic image acquisitions. Bioinformatics 25(11), 1463–1465. https://doi.org/10.1093/bioinformatics/btp184 (2009).
Acknowledgements
We would like to acknowledge RER for their help in preparation of brain slices used in this study. The authors acknowledge Nevzat Yaraş of iRiS Kinetics for valuable help in the fabrication of LIDs
Funding
The authors acknowledge funding from the U.S. National Institutes of Health (R01—AG075727, R01—AG075876 and 1R43NS135877-01) and National Science Foundation, NSF-TT PFI (2329817). We would also like to acknowledge support from the Boston University Office of Research—Kilichand fund.
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aslan, M., Gray, A.J., Packard, L. et al. Impact of uniform illumination in widefield microscopy and mesoscopy. Sci Rep 15, 25400 (2025). https://doi.org/10.1038/s41598-025-08754-0
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-025-08754-0