- Article
- Published:
- Jing Luo1 na1,
- Huali Yu1 na1,
- Xijing Yang ORCID: orcid.org/0000-0001-8451-20002,
- Dehui Wang ORCID: orcid.org/0000-0003-2444-71251,
- Bingyang Lu ORCID: orcid.org/0000-0002-7163-54551,
- Jiaxin Liu1,
- Jinlong Yang1,
- Yiming Zhang1,
- Shengjun Cheng3,
- Xianda Liu ORCID: orcid.org/0000-0001-6688-98083,
- Yupei Li ORCID: orcid.org/0000-0002-7364-37312,
- Fei Deng4,
- Guisen Li ORCID: orcid.org/0000-0003-1970-99794,
- Qiang Wei ORCID: orcid.org/0000-0001-5194-82623,
- Weifeng Zhao3,
- Baihai Su2,
- Changsheng Zhao ORCID: orcid.org/0000-0002-4619-34993 &
- …
- Xu Deng ORCID: orcid.org/0000-0002-9659-04171,5
Subjects
Abstract
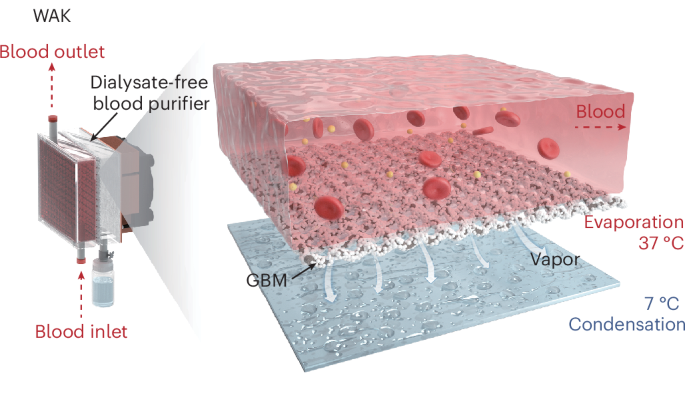
The wearable artificial kidney has emerged as a promising therapeutic alternative for end-stage renal disease due to its flexibility and portability, effectively clearing water and uremic toxins. However, conventional hemodialysis-based devices rely on liquid–liquid exchange and necessitate large quantities of dialysate to sustain a sufficient concentration gradient across the dialysis membrane, compromising portability and mobility. Here we develop a dialysate-free wearable artificial kidney prototype that uses a blood purifier for water removal, based on a vapor-driven liquid–gas phase transition, and integrates adsorption to remove uremic toxins. The system achieves a high water clearance flux of approximately 7 ml min−1 m−2 and successfully performs renal replacement therapy in rabbits with acute renal injury, efficiently removing water, creatinine and β2-microglobulin. With a current weight of less than 3.8 kg and potential for further engineering optimization, this dialysate-free wearable artificial kidney prototype supports the feasibility of practical portable blood purification, opening avenues for flexible and efficient nephropathy treatment.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Data availability
All data are available in the Article or the Supplementary Information. Source data are provided with this paper.
References
-
Bello, A. K. et al. Global Kidney Health Atlas: a report by the International Society of Nephrology on the global burden of end-stage kidney disease and capacity for kidney replacement therapy and conservative care across world countries and regions (International Society of Nephrology, 2019); https://www.theisn.org/wp-content/uploads/media/ISN%20Atlas_2023%20Digital.pdf
-
Cheng, W. et al. Survival outcomes of hemoperfusion and hemodialysis versus hemodialysis in patients with end-stage renal disease: a systematic review and meta-analysis. Blood Purificat. 51, 213–225 (2022).
-
Locatelli, F. et al. Mortality risk in patients on hemodiafiltration versus hemodialysis: a “real-world” comparison from the DOPPS. Nephrol. Dial. Transpl. 33, 683–689 (2018).
-
Powell, J. R. et al. Ten years experience of in-center thrice weekly long overnight hemodialysis. Clin. J. Am. Soc. Nephro. 4, 1097–1101 (2009).
-
Lacson, E. et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J. Am. Soc. Nephrol. 23, 687–695 (2012).
-
Nesrallah, G. E. et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J. Am. Soc. Nephrol. 23, 696–705 (2012).
-
McFarlane, P. A. et al. The quality of life and cost utility of home nocturnal and conventional in-center hemodialysis. Kidney Int. 64, 1004–1011 (2003).
-
Groth, T. et al. Wearable and implantable artificial kidney devices for end-stage kidney disease treatment: current status and review. Arti. Organs 47, 649–666 (2022).
-
Gura, V. et al. Technical breakthroughs in the wearable artificial kidney (WAK). Clin. J. Am. Soc. Nephro. 4, 1441–1448 (2009).
-
Davenport, A. et al. A wearable haemodialysis device for patients with end-stage renal failure: a pilot study. Lancet 370, 2005–2010 (2007).
-
Ramada, D. L. et al. Portable, wearable and implantable artificial kidney systems: needs, opportunities and challenges. Nat. Rev. Nephrol. 19, 481–490 (2023).
-
Gura, V. et al. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight 1, e86397 (2016).
-
Zamanzadeh, D. et al. Data-driven prediction of continuous renal replacement therapy survival. Nat. Commun. 15, 5440 (2024).
-
Luo, J., Fan, J.-B. & Wang, S. Recent progress of microfluidic devices for hemodialysis. Small 16, 1904076 (2020).
-
Savage, N. Could implantable artificial kidneys end the need for dialysis?. Nature 615, S12–S13 (2023).
-
Cassie, A. B. D. & Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 40, 546–551 (1944).
-
Cassie, A. B. D. Contact angles. Faraday Discuss. 3, 11–16 (1948).
-
Wang, D. et al. Design of robust superhydrophobic surfaces. Nature 582, 55–59 (2020).
-
Deng, X., Mammen, L., Butt, H.-J. & Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 335, 66–70 (2012).
-
Paven, M. et al. Super liquid-repellent gas membranes for carbon dioxide capture and heart-lung machines. Nat. Commun. 4, 2512 (2013).
-
Rosina, E. K. J., Šuta, D., Kolářová, H., Málek, J. & Krajči, L. Temperature dependence of blood surface tension. Physiol. Res. 56, S93–S98 (2007).
-
Song, X. et al. Transient blood thinning during extracorporeal blood purification via the inactivation of coagulation factors by hydrogel microspheres. Nat. Biomed. Eng. 5, 1143–1156 (2021).
-
Xu, T. et al. Self-anticoagulant sponge for whole blood auto-transfusion and its mechanism of coagulation factor inactivation. Nat. Commun. 14, 4875 (2023).
-
Pan, M. et al. Aggregation disruption induced multi-scale mediating strategy for anticoagulation in blood contacting devices. Adv. Mater. 35, 2412701 (2024).
-
Ji, H. et al. Antithrombotic coating with sheltered positive charges prevents contact activation by controlling factor XII–biointerface binding. Nat. Mater. 24, 626–636 (2025).
-
Brown, L. I. O.The Clausius-Clapeyron equation. J. Chem. Educ. 28, 428–428 (1951).
-
Tan, Y. et al. Liquid pressure guided superhydrophobic surfaces with adaptive adhesion and stability. Adv. Mater. 34, 2202167 (2022).
-
Butt, H.-J. et al. Design principles for superamphiphobic surfaces. Soft Matter 9, 418–428 (2013).
-
Gu, S. et al. Surface modification of polysulfones via one-pot ATRP and click chemistry: zwitterionic graft complex and their hemocompatibility. Fiber. Polym. 17, 161–165 (2016).
-
Tang, M. et al. Heparin-like surface modification of polyethersulfone membrane and its biocompatibility. J. Colloid Interf. Sci. 386, 428–440 (2012).
-
Ran, F. et al. Fabrication and cytocompatibility evaluation for blood-compatible polyethersulfone membrane modified by a synthesized poly (vinyl pyrrolidone)-block-poly (acrylate-graft-poly(methyl methacrylate))-block -poly-(vinyl pyrrolidone). Polym. Adv. Technol. 27, 591–596 (2016).
-
Shakaib, M., Ahmad, I., Yunus, R. M. & Noor, M. Z. Preparation and characterization of modified nylon 66 membrane for blood purification. Int. J. Polym. Mater. 63, 80–85 (2013).
-
Lu, Q., Shi, W., Yang, H. & Wang, X. Nanoconfined water-molecule channels for high-yield solar vapor generation under weaker sunlight. Adv. Mater. 32, 2001544 (2020).
-
Rezus, Y. L. A. & Bakker, H. J. Effect of urea on the structural dynamics of water. Proc. Natl Acad. Sci. USA 103, 18417–18420 (2006).
-
Léon, I. et al. Looking for the elusive imine tautomer of creatinine: different states of aggregation studied by quantum chemistry and molecular spectroscopy. ChemPlusChem. 86, 1374–1386 (2021).
-
Chen, J. et al. Broad-spectrum clearance of lipopolysaccharides from blood based on a hemocompatible dihistidine polymer. ACS Appl. Mater. 15, 32251–32261 (2023).
-
Fan, J.-B. et al. Bioinspired microfluidic device by integrating a porous membrane and heterostructured nanoporous particles for biomolecule cleaning. ACS Nano 13, 8374–8381 (2019).
-
Shi, Z. et al. Specific clearance of lipopolysaccharide from blood based on Peptide bottlebrush polymer for sepsis therapy. Adv. Mater. 35, 2302560 (2023).
Acknowledgements
We gratefully acknowledge the help of the Experimental Animal Center of West China Hospital Sichuan University for the animal experiments. We are grateful for the hemoperfusion resin adsorbents provided by Chongqing Healthcom Blood Purification Equipment Research and Development Co., Ltd. We are grateful for the assistance provided by J. Chen in the design and fabrication of dialysate-free WAK prototype. We are grateful to R. Zhong of the Institute of Blood Transfusion (IBT), Chinese Academy of Medical Sciences (CAMS)/Peking Union Medical College (PUMC), for help in the characterization of hemocompatibility, especially the deformability of red blood cells. We sincerely thank the Analysis and Testing Center of the University of Electronic Science and Technology of China for the technical support and assistance provided in the surface morphology characterization of GBMs. We acknowledge funding from the National Natural Science Foundation of China (22325201 (X.D.), 22205033 (J.L.) and 22275028 (D.W.)).
Ethics declarations
Competing interests
D.W., J. Luo and X.D. report the submission of a provisional patent application (patent application number 202210934680.9, China) encompassing the technologies described.
Peer review
Peer review information
Nature Chemical Engineering thanks Maria Grazia De Angelis, Bozhi Tian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, J., Yu, H., Yang, X. et al. A dialysate-free wearable artificial kidney prototype driven by a liquid–gas phase transition. Nat Chem Eng (2026). https://doi.org/10.1038/s44286-026-00355-6
-
Received:
-
Accepted:
-
Published:
-
Version of record:
-
DOI: https://doi.org/10.1038/s44286-026-00355-6