Dayan, C. M., Korah, M., Tatovic, D., Bundy, B. N. & Herold, K. C. Changing the Landscape for Type 1 Diabetes: The First Step to Prevention. Lancet 394, 1286–1296 (2019).
Gregory, A. et al. Global Incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modeling study. 10:741-760 (2022).
Beik, P. et al. Prevention of Type 1 Diabetes: Past Experiences and Future Opportunities. J. Clin. Med. 9, 2805 (2020).
Draznin, B. et al. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care 2022, S17–S38 (2020).
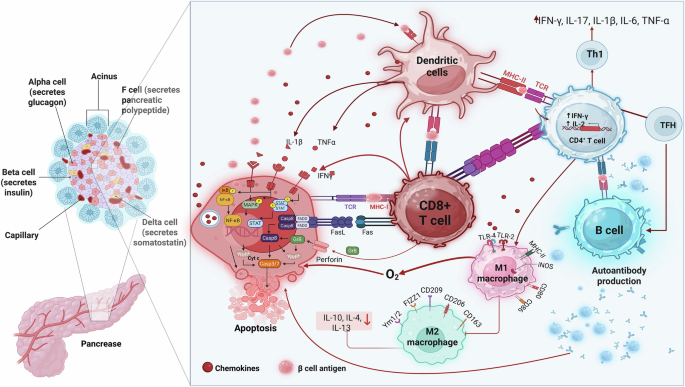
Herold, K.C., et al The immunology of type 1 diabetes. Nat Rev Immunol. 2. https://doi.org/10.1038/s41577-023-00985-4. (2024).
Colli, M. L., Szymczak, F. & Eizirik, D. L. Molecular Footprints of the Immune Assault on Pancreatic Beta Cells in Type 1 Diabetes. Front. Endocrinol. 11, 568446 (2020).
Lehuen, A., Diana, J., Zaccone, P. & Cooke, A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol 10, 501–513 (2010).
Rodriguez-Calvo, T., Richardson, S. J. & Pugliese, A. Pancreas Pathology During the Natural History of Type 1 Diabetes. Curr Diab Rep. 18, 124 (2018).
Carré, A., Richardson, S. J., Larger, E. & Mallone, R. Presumption of guilt for T cells in type 1 diabetes: lead culprits or partners in crime depending on age of onset. Diabetologia. Jan 64, 15–25 (2021).
Warshauer, J. T., Bluestone, J. A. & Anderson, M. S. New Frontiers in the Treatment of Type 1 Diabetes. Cell Metab. 31, 46–61 (2020).
Shilleh, A. H. & Russ, H. A. Cell Replacement Therapy for Type 1 Diabetes Patients: Potential Mechanisms Leading to Stem-Cell-Derived Pancreatic β cell Loss upon Transplant. Cells 12, 698 (2023).
Erickson, G. R. et al. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochemical and Biophysical Research Communications 290, 763–769 (2002).
Watt, F. M. & Hogan, B. L. Out of Eden: stem cells and their niches. Science 287, 1427–1430 (2000).
Takahashi, H., Sakata, N., Yoshimatsu, G., Hasegawa, S. & Kodama, S. Regenerative and Transplantation Medicine: Cellular Therapy Using Adipose Tissue- Derived Mesenchymal Stromal Cells for Type 1 Diabetes Mellitus. J Clin Med. 8, 249 (2019).
Shen, Z. et al. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front Immunol. 12, 749192 (2021).
Jasim, S. A. et al. Shining the light on clinical application of mesenchymal stem cell therapy in autoimmune diseases. Stem Cell Res Ther 13, 101 (2022).
Biarnés, M. et al. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 51, 66–72 (2002).
Czerwiec, K. et al. Adipose-Derived Mesenchymal Stromal Cells in Basic Research and Clinical Applications. Int J Mol Sci. 2023 24, 3888 (2023).
Ritter, A. et al. Subcutaneous and Visceral Adipose-Derived Mesenchymal Stem Cells: Commonality and Diversity. Cells. 8, 1288 (2019).
Gimble, J. M., Katz, A. J. & Bunnell, B. A. Adipose-derived stem cells for regenerative medicine. Circ Res 100, 1249–1260 (2007).
Mushahary, D. et al. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 93, 19–31 (2018). 2018 Jan.
Zuk, P. A. et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 7, 211–228 (2001).
Dave, S.D., Vanikar, A.V., Trivedi, H.L. Ex vivo generation of glucose sensitive insulin secreting mesenchymal stem cells derived from human adipose tissue. Indian J Endocrinol Metab. :S65-S69. (2012).
Mikłosz, A. & Chabowski, A. Adipose-derived Mesenchymal Stem Cells Therapy as a new Treatment Option for Diabetes Mellitus. J Clin Endocrinol Metab 108, 1889–1897 (2023).
Lee, J. et al. Differentiation of human adipose tissue-derived stem cells into aggregates of insulin-producing cells through the overexpression of pancreatic and duodenal homeobox gene-1. Cell Transplant. 22, 1053–1060 (2013).
Jaber, H., Issa, K., Eid, A., Saleh, F.A..The therapeutic effects of adipose-derived mesenchymal stem cells on obesity and its associated diseases in diet-induced obese mice. Sci Rep. 2021 11:6291. doi: 10.1038/s41598-021-85917-9. Erratum in: Sci Rep. 2021 11(1):12482. doi: 10.1038/s41598-021-91860-6 (2021)
Karaoz, E. et al. Adipose tissue-derived mesenchymal stromal cells efficiently differentiate into insulin-producing cells in pancreatic islet microenvironment both in vitro and in vivo. Cytotherapy. 15, 557–570 (2013).
Melief, S. M., Zwaginga, J. J., Fibbe, W. E. & Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2, 455–463 (2012). 2013 Jun.
Philippe B., et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT), Cytotherapy, Volume 15, Issue 6, 2013, Pages 641-648, ISSN 1465-3249, https://doi.org/10.1016/j.jcyt.2013.02.006.
Mohamed-Ahmed, S. et al. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther 9, 168 (2018).
Dmitrieva, R. I. et al. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: Differences and similarities. Cell Cycle. 11, 377–383 (2012).
Puissant, B. et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 129, 118–129 (2005).
Valencia, J. et al. Comparative analysis of the immunomodulatory capacities of human bone marrow- and adipose tissue-derived mesenchymal stromal cells from the same donor. Cytotherapy. 18, 1297–1311 (2016).
Yoshimura, H. et al. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. Mar 327, 449–462 (2007).
Yi, N. et al. Functional variation among mesenchymal stem cells derived from different tissue sources. PeerJ 12, e17616 (2024).
Liu, G. Y. et al. Adipose-Derived Mesenchymal Stem Cells Ameliorate Lipid Metabolic Disturbance in Mice. Stem Cells Transl Med. 5, 1162–1170 (2016).
Lin, C. S., Ning, H., Lin, G. & Lue, T. F. Is CD34 truly a negative marker for mesenchymal stromal cells. Cytotherapy 14, 1159–1163 (2012).
Hermiston, M. L., Xu, Z. & Weiss, A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 21, 107–137 (2001).
Sheng, M. et al. CD47-Mediated Hedgehog/SMO/GLI1 Signaling Promotes Mesenchymal Stem Cell Immunomodulation in Mouse Liver Inflammation. Hepatology. 74, 1560–1577 (2021).
Ceccarelli, S., Pontecorvi, P., Anastasiadou, E., Napoli, C. & Marchese, C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front Cell Dev Biol 8, 236 (2020).
Ezquer, F. et al. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells. 30, 1664–1674 (2012).
Nasef, A., Ashammakhi, N. & Fouillard, L. Immunomodulatory effect of mesenchymal stromal cells: possible mechanisms. Regen Med. 3, 531–546 (2008).
Ock, S. A. et al. Comparison of Immunomodulation Properties of Porcine Mesenchymal Stromal/Stem Cells Derived from the Bone Marrow, Adipose Tissue, and Dermal Skin Tissue. Stem Cells Int. 2016, 9581350 (2015).
Kocan, B., Maziarz, A., Tabarkiewicz, J., Ochiya, T. & Banaś-Ząbczyk, A. Trophic Activity and Phenotype of Adipose Tissue-Derived Mesenchymal Stem Cells as a Background of Their Regenerative Potential. Stem Cells Int 2017, 1653254 (2017).
Machado, C. D., da Silva Telles, P. D. & Nascimento, I. L. O. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 35, 62–67 (2013).
Li, Y. Y., Liu, H. H., Chen, H. L. & Li, Y. P. Adipose-derived mesenchymal stem cells ameliorate STZ-induced pancreas damage in type 1 diabetes. Biomed Mater Eng. 22, 97–103 (2012).
Rahavi, H. et al. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res. 2015, 878535 (2015).
Juedes, A. E. & von Herrath, M. G. Regulatory T-cells in type 1 diabetes. Diabetes Metab Res Rev. 20, 446–451 (2004).
Salomon, B. et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 12, 431–440 (2000). Apr.
Green, E. A., Gorelik, L., McGregor, C. M., Tran, E. H. & Flavell, R. A. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 100, 10878–10883 (2003).
Gregori, S., Giarratana, N., Smiroldo, S., Uskokovic, M. & Adorini, L. A1alpha,25- dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 51, 1367–1374 (2002).
Fousteri, G. et al. Subcutaneous insulin B:9-23/IFA immunisation induces Tregs that control late-stage prediabetes in NOD mice through IL-10 and IFNgamma. Diabetologia. 53, 1958–1970 (2010).
Grinberg-Bleyer, Y. et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 207, 1871–1878 (2010).
Bassi, ÊJ. et al. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes. 61, 2534–2545 (2012).
Lu, J., Liu, J., Li, L., Lan, Y. & Liang, Y. Cytokines in type 1 diabetes: mechanisms of action and immunotherapeutic targets. Clin Transl Immunology 9, e1122 (2020).
Banas, A. et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells 26, 2705–2712 (2008). no. 10, pp.2008.
Hervás-Salcedo, R. et al. Enhanced anti-inflammatory effects of mesenchymal stromal cells mediated by the transient ectopic expression of CXCR4 and IL10. Stem Cell Res Ther. 12, 124 (2021).
Li, J. X. et al. Current Clinical Progress of PD- 1/PD-L1 Immunotherapy and Potential Combination Treatment in Non-Small Cell Lung Cancer. Integr Cancer Ther. 18, 1534735419890020 (2019).
Shirley, M. Avelumab: A Review in Metastatic Merkel Cell Carcinoma. Target Oncol. 13, 409–416 (2018). 2018 Jun.
Friedlaender, A., Addeo, A., Banna, G. New emerging targets in cancer immunotherapy: the role of TIM3. ESMO Open. 2019 4(Suppl 3):e000497. (2019).
Solinas, C., Gu-Trantien, C. & Willard-Gallo, K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open. 5, e000544 (2020).
Leone, R. D., Lo, Y. C. & Powell, J. D. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J. 13, 265–272 (2015).
Tang, W., Chen, J., Ji, T. & Cong, X. TIGIT, a novel immune checkpoint therapy for melanoma. Cell Death Dis. 14, 466 (2023).
Paulos, C. M. & June, C. H. Putting the brakes on BTLA in T cell-mediated cancer immunotherapy. J Clin Invest. 120, 76–80 (2010).
Yang, Y., Islam, M. S., Hu, Y. & Chen, X. TNFR2: Role in Cancer Immunology and Immunotherapy. Immunotargets Ther. 10, 103–122 (2021).
Fujiwara, Y. et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat Rev. 110, 102461 (2022).
Meireson, A., Devos, M. & Brochez, L. IDO Expression in Cancer: Different Compartment, Different Functionality?. Front Immunol. 11, 531491 (2020).
Lau, A. P. Y., Khavkine, Binstock, S. S. & Thu, K. L. CD47: The Next Frontier in Immune Checkpoint Blockade for Non-Small Cell Lung Cancer. Cancers (Basel). 15, 5229 (2023).
Lian, S., Xie, X., Lu, Y. & Jia, L. Checkpoint CD47 Function On Tumor Metastasis And Immune Therapy. Onco Targets Ther. 12, 9105–9114 (2019).
Ivanova-Todorova, E. et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 126, 37–42 (2009).
Yi, T., Lee, D. S., Jeon, M. S., Kwon, S. W. & Song, S. U. Gene expression profile reveals that STAT2 is involved in the immunosuppressive function of human bone marrow-derived mesenchymal stem cells. Gene. 497, 131–139 (2012).
Saldanha-Araujo, F. et al. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 7, 66–74 (2011).
Cogdill, A. P., Andrews, M. C. & Wargo, J. A. Hallmarks of response to immune checkpoint blockade. Br J Cancer. 117, 1–7 (2017).
Murphy, K., Weaver, C., & Berg, L. Janeway’s immunobiology (9th ed.). https://www.ncbi.nlm.nih.gov/books/NBK10757/ (Garland Science, 2016).
Kawada-Horitani, E. et al. Human adipose-derived mesenchymal stem cells prevent type 1 diabetes induced by immune checkpoint blockade. Diabetologia. 65, 1185–1197 (2022).
Gaber, T. et al. CTLA-4 mediates inhibitory function of mesenchymal stem/stromal cells. Int. J. Mol. Sci. 19, 2312 (2018).
Kubli, S. P., Berger, T., Araujo, D. V., Siu, L. L. & Mak, T. W. Beyond immune checkpoint blockade: emerging immunological strategies. Nat. Rev. Drug Discov. 20, 899–919 (2021).
Yoneda, S. et al. T-lymphocyte infiltration to islets in the pancreas of a patient who developed type 1 diabetes after administration of immune checkpoint inhibitors. Diabetes Care. 42, e116–e118 (2019).
Hu, H., Zakharov, P. N., Peterson, O. J. & Unanue, E. R. Cytocidal macrophages in symbiosis with CD4 and CD8 T cells cause acute diabetes following checkpoint blockade of PD-1 in NOD mice. Proc. Natl. Acad. Sci. USA117, 31319–31330 (2020).
El-Sawah, S. G. et al. AD-MSCs and BMMSCs ameliorating effects on the metabolic and hepato-renal abnormalities in type 1 diabetic rats. Saudi J. Biol. Sci. 29, 1053–1060 (2022).
Nam, J. S. et al. Transplantation of insulin-secreting cells differentiated from human adipose tissue-derived stem cells into type 2 diabetes mice. Biochem. Biophys. Res. Commun. 443, 775–781 (2014).
Silva, I. B. B., Kimura, C. H., Colantoni, V. P. & Sogayar, M. C. Stem cells differentiation into insulin-producing cells (IPCs): recent advances and current challenges. Stem Cell Res Ther. 13, 309 (2022).
Brinkhof, B. et al. ALCAM (CD166) as a gene expression marker for human mesenchymal stromal cell characterisation. Gene X. 5, 100031 (2020).
Chhabra, P., Brayman, K.L. Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl. Med. 2, 328-336 (2013).
Su, W. et al. Diabetic microenvironment preconditioning of adipose tissue-derived mesenchymal stem cells enhances their anti- diabetic, anti-long-term complications, and anti-inflammatory effects in type 2 diabetic rats. Stem Cell Res. Ther. 13, 422 (2022).
Sen, S. et al. Genetic modification of human mesenchymal stem cells helps to reduce adiposity and improve glucose tolerance in an obese diabetic mouse model. Stem Cell Res. Ther. 6, 242 (2015).
Xue, B. et al. Mesenchymal stem cells modified by FGF21 and GLP1 ameliorate lipid metabolism while reducing blood glucose in type 2 diabetic mice. Stem Cell Res. Ther. 12, 133 (2021).
Kono, T. M. et al. Human adipose-derived stromal/stem cells protect against STZ-induced hyperglycemia: analysis of hASC-derived paracrine effectors. Stem Cells. 32, 1831–1842 (2014).
Badimon, L., Oñate, B. & Vilahur, G. Adipose-derived mesenchymal stem cells and their reparative potential in ischemic heart disease. Rev. Esp. Cardiol.68, 599–611 (2015). Jul.
Hoch, A. I., Binder, B. Y., Genetos, D. C. & Leach, J. K. Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One 7, e35579 (2012).
Sadat, S. et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem. Biophys. Res. Commun 363, 674–679 (2007).
Zhu, X. Y. et al. Transplantation of adipose-derived stem cells overexpressing hHGF into cardiac tissue. Biochem. Biophys. Res. Commun 379, 1084–1090 (2009).
Nakagami, H. et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 25, 2542–2547 (2005).
Kandasamy, M. et al. TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J. Cell Mol. Med. 18, 1444–1459 (2014).
Tsuji, W., Rubin, J. P. & Marra, K. G. Adipose-derived stem cells: implications in tissue regeneration. World J. Stem Cells 6, 312–321 (2014).
Rehman, J. et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 109, 1292–1298 (2004).
Mfopou, J. K., Willems, E., Leyns, L. & Bouwens, L. Expression of regulatory genes for pancreas development during murine embryonic stem cell differentiation. Int. J. Dev. Biol. 49, 915–922 (2005).
Kodama, S. Islet regeneration during the reversal of autoimmune diabetes in NOD mice. Science. 302, 1223–1227 (2003).
Zhang, H., Shen, Y., Kim, I. M., Weintraub, N. L. & Tang, Y. The impaired bioenergetics of diabetic cardiac microvascular endothelial cells. Front. Endocrinol. 12, 642857 (2021).
Forte, D. et al. Bone marrow mesenchymal stem cells support acute myeloid leukemia bioenergetics and enhance antioxidant defense and escape from chemotherapy. Cell Metab. 32, 829–843.e9 (2020).
Kajiyama, H., Hamazaki, T. S., Tokuhara, M., Masui, S. & Okabayashi, K. Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice. Int. J. Dev. Biol. 54, 699–705 (2010).
Kumar, M. A. et al. Extracellular vesicles as tools and targets in therapy for diseases. Sig. Transduct. Target Ther. 9, 27 (2024).
Soltani, S. et al. Extracellular vesicle therapy for type 1 diabetes. Front. Immunol. 13, 865782 (2022).
Tsukita, S. et al. MicroRNAs 106b and 222 improve hyperglycemia in a mouse model of insulin-deficient diabetes via pancreatic β-cell proliferation. EBioMedicine 15, 163–172 (2017).
Chen, J. et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting P38 MAPK phosphorylation. Stem Cell Res. Ther. 11, 97 (2020).
Cantaluppi, V. et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell transplantation 21, 1305–1320 (2012).
Mohammadi, M. R. et al. Exosome loaded immunomodulatory biomaterials alleviate local immune response in immunocompetent diabetic mice post islet xenotransplantation. Commun. Biol. 4, 685 (2021).
Nie, W. et al. Human mesenchymal-stem-cells- derived exosomes are important in enhancing porcine islet resistance to hypoxia. Xenotransplantation 25, e12405 (2018).
Keshtkar, S. et al. Exosomes derived from human mesenchymal stem cells preserve mouse islet survival and insulin secretion function. EXCLI J 19, 1064–1080 (2020).
Zhang, L. et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 11, 1562–1569 (2020).
Zhu, Y., Jia, Y., Wang, Y., Xu, J. & Chai, Y. Impaired bone regenerative effect of exosomes derived from bone marrow mesenchymal stem cells in type 1 diabetes. Stem Cells Transl. Med. 8, 593–605 (2019).
Liang, B. et al. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 10, 1410 (2019).
Liu, W. et al. Melatoninstimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther 11, 1756 (2020).
Villatoro, A. J. et al. Comparative analysis and characterization of soluble factors and exosomes from cultured adipose tissue and bone marrow mesenchymal stem cells in canine species. Veterinary immunology and immunopathology 208, 6–15 (2019).
Hoang, D. H. et al. Differential wound healing capacity of mesenchymal stem cell- derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and xeno-free condition. Front. Mol. Biosci. 7, 119 (2020).
Bari, E. et al. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine 14, 753–765 (2019).
Chance, T. C. et al. The effects of cell type and culture condition on the procoagulant activity of human mesenchymal stromal cell-derived extracellular vesicles. J. Trauma Acute Care Surg. 87, S74–S82 (2019).
Han, Y. et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Sig. Transduct. Target The.r 7, 92 (2022).
Bekeredjian-Ding, I. et al. Tumour-derived prostaglandin E and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha. Immunology. 128, 439–450 (2009).
Horiguchi, M., Okada, Y., Turudome, Y. & Ushijima, K. Exosome degeneration in mesenchymal stem cells derived from patients with type 1 diabetes mellitus. Int. J. Mol. Sci. 22, 10906 (2021).
Kulaj, K. et al. Adipocyte-derived extracellular vesicles increase insulin secretion through transport of insulinotropic protein cargo. Nat Commun. 14, 709 (2023).
Gesmundo, I. et al. Adipocyte-derived extracellular vesicles regulate survival and function of pancreatic β cells. JCI Insight. 6, e141962 (2021).
Nojehdehi, S. et al. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell Biochem. 119, 9433–9443 (2018).
Rahman, M. M. et al. CD13 promotes mesenchymal stem cell-mediated regeneration of ischemic muscle. Front Physiol. 4, 402 (2014).
Wu, C. C. et al. CD146+ mesenchymal stem cells display greater therapeutic potential than CD146– cells for treating collagen-induced arthritis in mice. Stem Cell Res. Ther. 7, 23 (2016).
Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells. 1, 1–7 (2008).
Dang, L. T. et al. Intravenous infusion of human adipose tissue-derived mesenchymal stem cells to treat type 1 diabetic mellitus in mice: an evaluation of grafted cell doses. Adv. Exp. Med. Biol. 1083, 145–156 (2017).
Barbash, I. M. et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 108, 863–868 (2003).
Rochefort, G. Y. et al. Influence of hypoxia on the domiciliation of mesenchymal stem cells after infusion into rats: possibilities of targeting pulmonary artery remodeling via cells therapies. Respir Res. 6, 125 (2005).
Baas, J., Senninger, N. & Elser, H. Das retikuloendotheliale System. Eine Ubersicht über Funktion, Pathologie und neuere Messmethoden [The reticuloendothelial system. An overview of function, pathology and recent methods of measurement]. Z Gastroenterol. 32, 117–123 (1994).
Hashemi, S. M., Hassan, Z. M., Hossein-Khannazer, N., Pourfathollah, A. A. & Soudi, S. Investigating the route of administration and efficacy of adipose tissue-derived mesenchymal stem cells and conditioned medium in type 1 diabetic mice. Inflammopharmacology. 28, 585–601 (2020).
Wang, M. et al. Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Sci. Rep. 6, 30696 (2016).
Arzouni, A. A. et al. Mesenchymal stromal cells improve human islet function through released products and extracellular matrix. Clin. Sci. 131, 2835–2845 (2017).
Leibacher, J. & Henschler, R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res. Ther. 7, 7 (2016).
Schrepfer, S. et al. Stem cell transplantation: the lung barrier. Transplant. Proc. 39, 573–576 (2007).
Schroder, C., Khatri, R., Petry, S.F., Linn, T. Class I and II histone deacetylase inhibitor LBH589 promotes endocrine differentiation in bone marrow derived human mesenchymal stem cells and suppresses uncontrolled proliferation. Exp. Clin. Endocrinol. Diabetes. 129, 357-364 (2020).
Wang, H. et al. Autologous mesenchymal stem cell and islet cotransplantation: safety and efficacy. Stem Cells Transl. Med. 7, 11–19 (2018).
Lee, R. H. et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 5, 54–63 (2009).
Khatri, R., Petry, S. F. & Linn, T. Intrapancreatic MSC transplantation facilitates pancreatic islet regeneration. Stem Cell Res. Ther. 12, 121 (2021).
Steingen, C. et al. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J. Mol. Cell Cardiol. 44, 1072–1084 (2008).
Yarani, R. et al. Intra-arterial delivery of mesenchymal stromal cells maintains glycemic regulation in streptozotocin-treated rats. Cytotherapy 2024, 26 (2024).
Yaochite, J. N. et al. Therapeutic efficacy and biodistribution of allogeneic mesenchymal stem cells delivered by intrasplenic and intrapancreatic routes in streptozotocin-induced diabetic mice. Stem Cell Res. Ther. 6, 31 (2015).
Gabbay, M. A. et al. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual β cell function in new-onset type 1 diabetes mellitus. Arch. Pediatr. Adolesc. Med. 166, 601–607 (2012).
Ministry of Food and Drug Safety, Republic of Korea. (Cell therapy products) ANTEROGEN. MFDS. Published February 15, 2017. Accessed August 12, 2024. https://www.mfds.go.kr/eng/brd/m_30/view.do?seq=71337
Scott, L. J. Darvadstrocel: a review in treatment-refractory complex perianal fistulas in Crohn’s Disease. BioDrugs. 32, 627–634 (2018).
European Medicines Agency. Alofisel: EPAR—Product Information. Published May 31, 2018. Accessed August 12, 2024, https://www.ema.europa.eu/en/documents/product-information/alofisel-epar-product-information_en.pdf
Kostecka, A. et al. Adipose-derived mesenchymal stromal cells in clinical trials: Insights from single-cell studies. Life Sci. 351, 122761 (2024).
Wright, A., Arthaud-Day, M. L. & Weiss, M. L. Therapeutic use of mesenchymal stromal cells: The need for inclusive characterization guidelines to accommodate all tissue sources and species. Front. Cell Dev. Biol. 9, 632717 (2021).
Bourin, P. et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 15, 641–648 (2013).
Lan, T., Luo, M. & Wei, X. Mesenchymal stem/stromal cells in cancer therapy. J. Hematol. Oncol. 14, 195 (2021).
King, N. M. & Perrin, J. Ethical issues in stem cell research and therapy. Stem Cell Res. Ther. 5, 85 (2014).
Lappin, T. & Cheng, T. An urgent need for standardization of stem cells and stem cell-derived products toward clinical applications. Stem Cells Transl. Med. 10, S1–S3 (2021).
Firriolo, J. M., Condé-Green, A. & Pu, L. L. Q. Fat grafting as regenerative surgery: a current review. Plast. Reconstr. Surg. 150, 1340e–1347e (2022).
Fiorello, M. L., Treweeke, A. T., Macfarlane, D. P. & Megson, I. L. The impact of glucose exposure on bioenergetics and function in a cultured endothelial cell model and the implications for cardiovascular health in diabetes. Sci. Rep. 10, 19547 (2020).
Ahmed, L. & Al-Massri, K. New approaches for enhancement of the efficacy of mesenchymal stem cell-derived exosomes in cardiovascular diseases. Tissue Eng. Regen. Med. 19, 1129–1146 (2022).
Tremolada, C. et al. Adipose tissue and mesenchymal stem cells: state of the art and lipogems® technology development. Curr. Stem Cell Rep. 2, 304–312 (2016).
Chen, S., et al. (2022). Preconditioning and engineering strategies for improving the efficacy of mesenchymal stem cell-derived exosomes in cell-free therapy. Stem Cells Int. 2022, 1779346 (2022).
Alagesan, S. et al. Enhancement strategies for mesenchymal stem cells and related therapies. Stem Cell Res. Ther. 13, 75 (2022).
Hazrati, A. et al. Therapeutic and immunomodulatory potentials of mesenchymal stromal/stem cells and immune checkpoints related molecules. Biomark Res. 12, 35 (2024).
Magna, M. & Pisetsky, D. S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol. Med. 20, 138–146 (2014).
Scherberich, A., Di Maggio, N. D. & McNagny, K. M. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J. Stem Cells. 5, 1–8 (2013).
Deschepper, M. et al. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J. Cell Mol. Med. 15, 1505–1514 (2011).
Tsai, C. C. et al. Benefits of hypoxic culture on bone marrow multipotent stromal cells. Am. J. Blood Res. 2, 148–159 (2012).
Park, W. S. et al. Strategies to enhance paracrine potency of transplanted mesenchymal stem cells in intractable neonatal disorders. Pediatr. Res. 83, 214–222 (2018).
Patel, D. B. et al. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng. Transl. Med. 2, 170–179 (2017).
Huang, S. et al. Increased CD34 in pancreatic islet negatively predict islet β-cell decrease in type1 diabetes model. Front. Physiol. 13, 1032774 (2022).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Sorgun, O. & Erbaş, O. Adipose-derived mesenchymal stem cells mitigate methotrexate-induced liver cirrhosis (fibrosis) model. Eur. Rev. Med. Pharmacol. Sci. 27, 11882–11889 (2023).
Yang, Y. et al. CD29 of human umbilical cord mesenchymal stem cells is required for expansion of CD34(+) cells. Cell Prolif. 47, 596–603 (2014).
Ode, A. et al. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur Cell Mater. 22, 26–42 (2011).
Zhou, L. et al. Role of CD44 in increasing the potency of mesenchymal stem cell extracellular vesicles by hyaluronic acid in severe pneumonia. Stem Cell Res. Ther. 12, 293 (2021).
Li, C. et al. Adipose mesenchymal stem cell-derived exosomes promote wound healing through the WNT/β-catenin signaling pathway in dermal fibroblasts. Stem Cell Rev. Rep. 18, 2059–2073 (2022).
Monguió-Tortajada, M. et al. Mesenchymal stem cells induce expression of CD73 in human monocytes in vitro and in a swine model of myocardial infarction in vivo. Front. Immunol. 8, 1577 (2017).
Moraes, D. A. et al. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res. Ther. 9, 97 (2016).
Thitilertdecha, P. et al. Extensive characterization of mesenchymal stem cell marker expression on freshly isolated and in vitro expanded human adipose-derived stem cells from breast cancer patients. Stem Cells Int. 2020, 8237197 (2020).
Suga, H. et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 18, 1201–1210 (2009).
Radu, P. et al. CD34—structure, functions and relationship with cancer stem cells. Medicina. 59, 938 (2023).
Sattler, C. et al. Inhibition of T-cell proliferation by murine multipotent mesenchymal stromal cells is mediated by CD39 expression and adenosine generation. Cell Transplant. 20, 1221–1230 (2011).
Shahbaz, S. et al. CD71+VISTA+ erythroid cells promote the development and function of regulatory T cells through TGF-β. PLoS Biol 16, e2006649 (2018).
Balazs, A. B., Fabian, A. J., Esmon, C. T. & Mulligan, R. C. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 107, 2317–2321 (2006).
Kachroo, U., Ramasamy, B. & Vinod, E. Evaluation of CD49e as a distinguishing marker for human articular cartilage derived chondroprogenitors. Knee. 27, 833–837 (2020).
Mohammadi, A. et al. Immunomodulatory and protective effects of adipose tissue-derived mesenchymal stem cells in an allograft islet composite transplantation for experimental autoimmune type 1 diabetes. Immunol Lett. 188, 21–31 (2017). Aug.
Rolfo, C. et al. Immunotherapy in NSCLC: a promising and revolutionary weapon. Adv. Exp. Med. Biol. 995, 97–125 (2017).
Wu, G. Therapeutic effects of pembrolizumab combined with paclitaxel and cisplatin chemotherapy on advanced non-squamous non-small cell lung cancer and influencing factors. Indian J. Pharm. Sci. 83, 120–126 (2021).
Migden, M. R. et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N. Engl. J. Med. 379, 341–351 (2018).
Paz-Ares, L. et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann. Oncol. 31, 798–806 (2018).
Li, X., Xu, Z., Cui, G., Yu, L. & Zhang, X. BTLA expression in stage I-III non- small-cell lung cancer and its correlation with PD-1/PD-L1 and clinical outcomes. Onco Targets Ther. 13, 215–224 (2020).
Peggs, K. S., Quezada, S. A. & Allison, J. P. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol. Rev. 224, 141–165 (2008).
Zhu, L.L., et al. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia. 2018. https://doi.org/10.1111/and.12871 (2017).