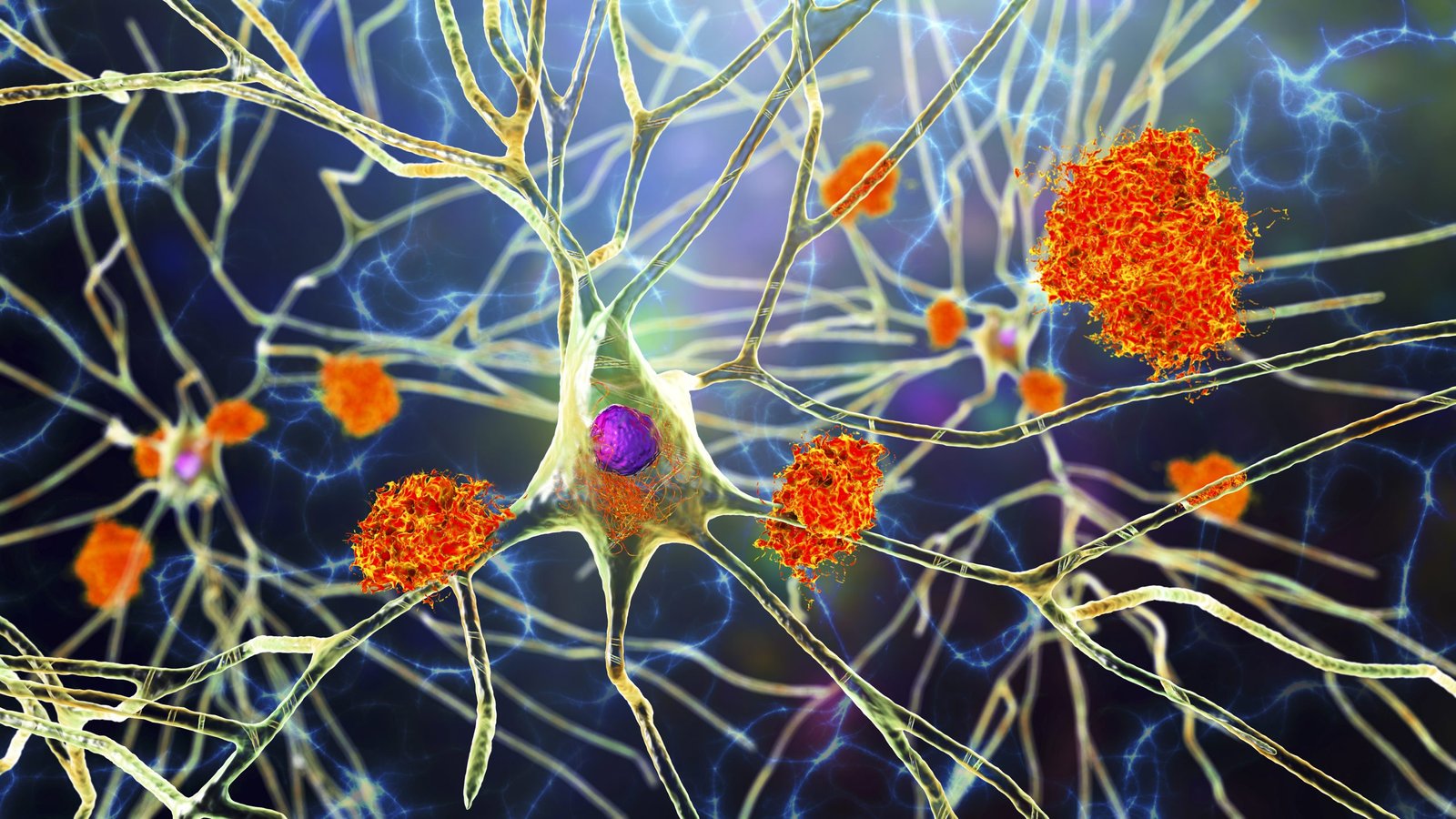
Scientists at UC San Francisco and at Gladstone Institutes have identified a combination of two anticancer drugs that the researchers suggest may reverse the changes that occur in the brain during Alzheimer’s disease, potentially slowing or even reversing its symptoms.
The team first analyzed how Alzheimer’s disease altered gene expression in single cells in the human brain. They then looked for existing FDA-approved drugs that would reverse the gene expression changes in neurons and in other types of brain cells called glia, all of which are damaged or altered in Alzheimer’s disease.
Next, the team analyzed millions of electronic medical records to show that patients who took some of these drugs as part of their treatment for other conditions were less likely to get Alzheimer’s disease. They tested a combination of the two top drugs, letrozole and irinotecan—which are both anticancer drugs—in a mouse model of Alzheimer’s. The results showed that the drug combination reduced brain degeneration in the mice and even restored their ability to remember.
“Alzheimer’s disease comes with complex changes to the brain, which has made it tough to study and treat, but our computational tools opened up the possibility of tackling the complexity directly,” said Marina Sirota, PhD, the interim director of the UCSF Bakar Computational Health Sciences Institute, professor of pediatrics, “We’re excited that our computational approach led us to a potential combination therapy for Alzheimer’s based on existing FDA-approved medications.”
Sirota is co-senior author of the team’s published paper in Cell, titled “Cell-type-directed network-correcting combination therapy for Alzheimer’s disease,” in which the team concluded, “…by targeting molecular signatures and clinical features derived from real world individuals, this study demonstrates the potential for AI enabled precision medicine leveraging large-scale multimodal personalized measurements…Together, these findings provide strong clinical and mechanistic support for the potential efficacy of letrozole and irinotecan as a combination therapy for AD.”
Alzheimer’s disease (AD) is a neurodegenerative disorder that results in progressive memory loss, behavior changes, and cognitive and motor deficits, the authors explained. They cite figures indicating that 50 million people are currently living with AD or other related dementias worldwide, and that this number is projected to triple by 2050. However, they noted, decades of research have only produced two FDA-approved drugs, neither of which can meaningfully slow this decline. “Despite rigorous preclinical and clinical research efforts, AD drug development faces significant challenges, with a 98% failure rate in recent decades,” the team stated.
Co-senior author Yadong Huang, MD, PhD, senior investigator and director of the Center for Translational Advancement at Gladstone, professor of neurology and pathology at UCSF, further explained, “Alzheimer’s is likely the result of numerous alterations in many genes and proteins that, together, disrupt brain health. This makes it very challenging for drug development—which traditionally produces one drug for a single gene or protein that drives disease.” And as the authors noted in their paper, “Current therapeutic options are mostly limited to symptom-managing treatments…Urgent action is needed to develop more effective and accessible treatments.”
To address the challenges associated with developing treatments for Alzheimer’s disease, the researchers introduced what they described as “…a cell-type-specific, multi-target drug discovery strategy grounded in human data and real-world evidence.” This drug screening strategy, they suggest, is entirely driven by human data, “…utilizing large-scale omic datasets from post-mortem brains, a drug perturbation library generated in human cell lines and clinical records encompassing millions of individuals, thereby maximizing the chance of clinical translation.”
The team took publicly available data from three studies of the Alzheimer’s brain that measured single-cell gene expression in brain cells from deceased donors with or without Alzheimer’s disease. They used this data to produce gene expression signatures for Alzheimer’s disease in neurons and glia.
The researchers compared these signatures with those found in the Connectivity Map, a database of results from testing the effects of thousands of drugs on gene expression in human cells. They found that out of 1,300 drugs, 86 reversed the Alzheimer’s disease gene expression signature in one cell type, and 25 reversed the signature in several cell types in the brain. But just 10 had already been approved by the FDA for use in humans. “Given the unmet need for disease-modifying treatments, drug repurposing has gained interest due to its faster development, lower costs, and improved safety,” they suggested in their paper. “In addition, technical advancements in mining large-scale databases offer new opportunities for discovering promising candidates.”
Poring through records housed in the UC Health Data Warehouse, which includes anonymized health information on 1.4 million people over the age of 65, the group found that several of these drugs seemed to have reduced the risk of developing Alzheimer’s disease over time.
“Thanks to all these existing data sources, we went from 1,300 drugs, to 86, to 10, to just 5,” said lead author Yaqiao Li, PhD, a former UCSF graduate student in Sirota’s lab who is now a postdoctoral scholar in Huang’s lab at Gladstone. “In particular, the rich data collected by all the UC health centers pointed us straight to the most promising drugs. It’s kind of like a mock clinical trial.”
Li, Huang, and Sirota selected two cancer drugs out of the top five drug candidates for laboratory testing. They predicted one drug, letrozole, would remedy Alzheimer’s in neurons, and another, irinotecan, would help glia. Letrozole is usually used to treat breast cancer, while irinotecan is usually used to treat colon and lung cancer.
The team tested the drug combination in a mouse model of aggressive Alzheimer’s disease with multiple disease-related mutations. As the mice aged, symptoms resembling Alzheimer’s emerged, and they were treated with one or both drugs.
The investigators found that the combination of the two cancer drugs reversed multiple aspects of Alzheimer’s in the animal model. It undid the gene expression signatures in neurons and glia that had emerged as the disease progressed. It reduced both the formation of toxic clumps of proteins and brain degeneration. And, importantly, it restored memory.
“Preclinical validation in an AD mouse model demonstrated that the proposed drug combination significantly improved memory deficits and alleviated AD-related pathologies, whereas the single drugs alone showed limited or no efficacy, highlighting the superior therapeutic potential of the combination treatment,” they wrote. “The successful in vivo validation underscores the power of multi-cell-type network-correction therapies in effectively treating AD, highlighting the promise of our human-data-driven, cell-specific drug discovery approach to develop comprehensive therapies for complex diseases.”
“It’s so exciting to see the validation of the computational data in a widely used Alzheimer’s mouse model,” added Huang, who expects the research to advance soon to a clinical trial so the team can directly test the combination therapy in Alzheimer’s patients.
Sirota noted, “If completely independent data sources, such as single-cell expression data and clinical records, guide us to the same pathways and the same drugs, and then resolve Alzheimer’s in a genetic model, then maybe we’re onto something. We’re hopeful this can be swiftly translated into a real solution for millions of patients with Alzheimer’s.”
In their discussion the authors also suggested that their study “… paves the way for precision medicine leveraging AI and large-scale patient-level data.”
The post Alzheimer’s Mouse Model Shows Memory Boost from Anticancer Drug Combo appeared first on GEN – Genetic Engineering and Biotechnology News.