- Research Briefing
- Published:
Subjects
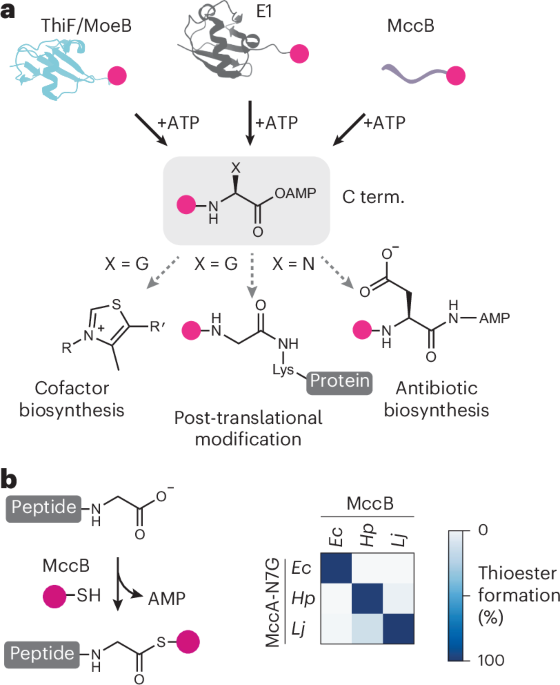
Current tools for protein bioconjugation at the C-terminal have limited yields. Now, an enzymatic strategy for ATP-dependent activation of protein and peptide C termini has been developed. This versatile tool can be deployed for synthesis of C-terminal thioesters that enhance the yield and accessibility of diverse protein bioconjugation methods.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

References
-
Stephanopoulos, N. & Francis, M. B. Choosing an effective protein bioconjugation strategy. Nat. Chem. Biol. 7, 876–884 (2011). A review article that presents considerations and strategies for protein bioconjugation.
-
Walsh, C. T., Tu, B. P. & Tang, Y. Eight kinetically stable but thermodynamically activated molecules that power cell metabolism. Chem. Rev. 118, 1460–1494 (2018). This review article describes the chemical logic by which cellular metabolism is coupled to energetic driving forces.
-
Thompson, R. E. & Muir, T. W. Chemoenzymatic semisynthesis of proteins. Chem. Rev. 120, 3051–3126 (2020). This review article surveys chemical and enzymatic strategies that can be used for protein semisynthesis.
-
Burroughs, A. M., Iyer, L. M. & Aravind, L. Natural history of the E1‐like superfamily: Implication for adenylation, sulfur transfer, and ubiquitin conjugation. Proteins Struct. Funct. Bioinform. 75, 895–910 (2009). This paper describes the functional diversity and evolutionary history of the ThiF enzyme superfamily.
-
Roush, R. F., Nolan, E. M., Löhr, F. & Walsh, C. T. Maturation of an Escherichia coli ribosomal peptide antibiotic by ATP-consuming N−P bond formation in microcin C7. J. Am. Chem. Soc. 130, 6055–6055 (2008). This paper describes the enzymatic strategy used by MccB for ATP-dependent N–P bond formation via a C-terminal adenylate intermediate.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This is a summary of: Frazier, C. L. et al. Engineered reactivity of a bacterial E1-like enzyme enables ATP-driven modification of protein and peptide C termini. Nat. Chem. https://doi.org/10.1038/s41557-025-01871-3 (2025).
Rights and permissions
About this article
Cite this article
An enzyme for high-yield, ATP-driven C-terminal thioester generation. Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01872-2
-
Published:
-
DOI: https://doi.org/10.1038/s41557-025-01872-2