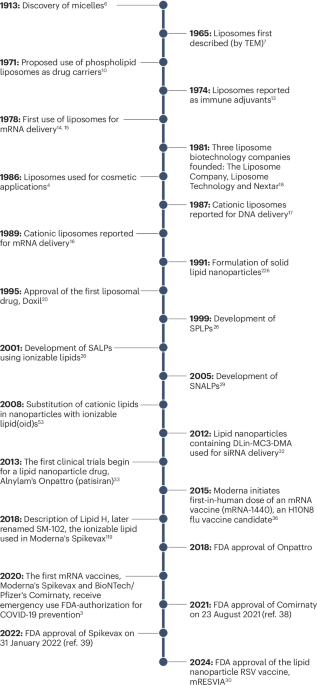
References
-
Diercks, C. S., Dik, D. A. & Schultz, P. G. Adding new chemistries to the central dogma of molecular biology. Chem 7, 2883–2895 (2021).
-
Schoenmaker, L. et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int. J. Pharm. 601, 120586 (2021).
-
Pardi, N. & Krammer, F. mRNA vaccines for infectious diseases — advances, challenges and opportunities. Nat. Rev. Drug Discov. 23, 838–861 (2024).
-
Tenchov, R., Bird, R., Curtze, A. E. & Zhou, Q. Lipid nanoparticles from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 15, 16982–17015 (2021).
-
Giuliano, C. B., Cvjetan, N., Ayache, J. & Walde, P. Multivesicular vesicles: preparation and applications. ChemSystChem 3, e2000049 (2021).
-
Vincent, B. McBain and the centenary of the micelle. Adv. Colloid Interf. Sci. 203, 51–54 (2014).
-
Bangham, A. D. & Horne, R. W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 8, 660–668 (1964).
-
Bangham, A. D., Standish, M. M. & Watkins, J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 13, 238–252 (1965).
-
Hope, M. J., Bally, M. B., Webb, G. & Cullis, P. R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta Biomembr. 812, 55–65 (1985).
-
Gregoriadis, G., Leathwood, P. D. & Ryman, B. E. Enzyme entrapment in liposomes. FEBS Lett. 14, 95–99 (1971).
-
Gregoriadis, G. Drug entrapment in liposomes. FEBS Lett. 36, 292–296 (1973).
-
Gregoriadis, G. & Allison, A. C. Entrapment of proteins in liposomes prevents allergic reactions in pre-immunised mice. FEBS Lett. 45, 71–74 (1974).
-
Allison, A. C. & Gregoriadis, G. Liposomes as immunological adjuvants. Nature 252, 252–252 (1974).
-
Ostro, M. J., Giacomoni, D., Lavelle, D., Paxton, W. & Dray, S. Evidence for translation of rabbit globin mRNA after liposome-mediated insertion into a human cell line. Nature 274, 921–923 (1978).
-
Dimitriadis, G. J. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature 274, 923–924 (1978).
-
Malone, R. W., Felgner, P. L. & Verma, I. M. Cationic liposome-mediated RNA transfection. Proc. Natl Acad. Sci. USA 86, 6077–6081 (1989).
-
Felgner, P. L. et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl Acad. Sci. USA 84, 7413–7417 (1987). This article reports lipofection, establishing that cationic lipids can deliver nucleic acids into mammalian cells.
-
Gregoriadis, G. Liposomology: delivering the message. J. Liposome Res. 28, 1–4 (2018).
-
Liu, P., Chen, G. & Zhang, J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules 27, 1372 (2022).
-
Barenholz, Y. Doxil® — the first FDA-approved nano-drug: lessons learned. J. Control. Release 160, 117–134 (2012).
-
Giordani, S., Marassi, V., Zattoni, A., Roda, B. & Reschiglian, P. Liposomes characterization for market approval as pharmaceutical products: analytical methods, guidelines and standardized protocols. J. Pharm. Biomed. Anal. 236, 115751 (2023).
-
Taniguchi, H. et al. Liposomal amphotericin B formulation displaying lipid-modified chitin-binding domains with enhanced antifungal activity. Mol. Pharm. 19, 3906–3914 (2022).
-
Abraham, S. A. et al. The liposomal formulation of doxorubicin. Methods Enzymol. 391, 71–97 (2005).
-
Filion, M. C. & Phillips, N. C. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim. Biophys. Acta Biomembr. 1329, 345–356 (1997).
-
Tousignant, J. D. et al. Comprehensive analysis of the acute toxicities induced by systemic administration of cationic lipid:plasmid DNA complexes in mice. Hum. Gene Ther. 11, 2493–2513 (2000).
-
Wheeler, J. J. et al. Stabilized plasmid–lipid particles: construction and characterization. Gene Ther. 6, 271–281 (1999).
-
Maurer, N. et al. Spontaneous entrapment of polynucleotides upon electrostatic interaction with ethanol-destabilized cationic liposomes. Biophys. J. 80, 2310–2326 (2001).
-
Semple, S. C. et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta Biomembr. 1510, 152–166 (2001).
-
Morrissey, D. V. et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 23, 1002–1007 (2005).
-
Mullard, A. FDA approves mRNA-based RSV vaccine. Nat. Rev. Drug Discov. 23, 487 (2024).
-
Cullis, P. R. & Felgner, P. L. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug Discov. 23, 709–722 (2024).
-
Jayaraman, M. et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Edn 51, 8529–8533 (2012). This article reports a rational design approach to identifying DLin-MC3-DMA, the ionizable lipid that enabled the first siRNA–lipid-nanoparticle therapy to be approved by the FDA.
-
Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018).
-
Wood, H. FDA approves patisiran to treat hereditary transthyretin amyloidosis. Nat. Rev. Neurol. 14, 570–570 (2018).
-
Sabnis, S. et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26, 1509–1519 (2018).
-
Feldman, R. A. et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 37, 3326–3334 (2019).
-
Haynes, B. F. A new vaccine to battle covid-19. N. Engl. J. Med. 384, 470–471 (2021).
-
Mullard, A. Pfizer’s COVID-19 vaccine secures first full FDA approval. Nat. Rev. Drug Discov. 20, 728 (2021).
-
Gilbert, P. B. et al. A covid-19 milestone attained — a correlate of protection for vaccines. N. Engl. J. Med. 387, 2203–2206 (2022).
-
Santel, A. et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 13, 1222–1234 (2006).
-
Sago, C. D. et al. High-throughput in vivo screen of functional mRNA delivery identifies nanoparticles for endothelial cell gene editing. Proc. Natl Acad. Sci. USA 115, E9944–E9952 (2018).
-
Zhi, D. et al. The headgroup evolution of cationic lipids for gene delivery. Bioconj. Chem. 24, 487–519 (2013).
-
Eygeris, Y., Gupta, M., Kim, J. & Sahay, G. Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 55, 2–12 (2022).
-
Hajj, K. A. et al. A potent branched-tail lipid nanoparticle enables multiplexed mRNA delivery and gene editing in vivo. Nano Lett. 20, 5167–5175 (2020).
-
Whitehead, K. A. et al. Synergistic silencing: combinations of lipid-like materials for efficacious siRNA delivery. Mol. Ther. 19, 1688–1694 (2011).
-
Hou, X. et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 15, 41–46 (2020).
-
Alabi, C. A. et al. Multiparametric approach for the evaluation of lipid nanoparticles for siRNA delivery. Proc. Natl Acad. Sci. USA 110, 12881–12886 (2013).
-
Carrasco, M. J. et al. Ionization and structural properties of mRNA lipid nanoparticles influence expression in intramuscular and intravascular administration. Commun. Biol. 4, 956 (2021).
-
Cullis, P. R. & Hope, M. J. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25, 1467–1475 (2017).
-
Kulkarni, J. A. et al. On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano 12, 4787–4795 (2018). This article reports the use of cryo-electron microscopy to reveal that lipid nanoparticles form a nanostructured core–shell architecture, clarifying how lipids organize around RNA.
-
Evers, M. J. W. et al. State-of-the-art design and rapid-mixing production techniques of lipid nanoparticles for nucleic acid delivery. Small Methods 2, 1700375 (2018).
-
Zhang, C. et al. Antibiotic-derived lipid nanoparticles to treat intracellular Staphylococcus aureus. ACS Appl. Bio Mater. 2, 1270–1277 (2019).
-
Akinc, A. et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 26, 561–569 (2008). This article reports an accelerated discovery approach for ionizable lipids for RNA delivery using combinatorial chemistry.
-
Lee, S. M. et al. A systematic study of unsaturation in lipid nanoparticles leads to improved mRNA transfection in vivo. Angew. Chem. Int. Edn 60, 5848–5853 (2021).
-
Patel, S., Ryals, R. C., Weller, K. K., Pennesi, M. E. & Sahay, G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J. Control. Release 303, 91–100 (2019).
-
Petersen, D. M. S. et al. Branched-tail lipid nanoparticles for intravenous mRNA delivery to lung immune, endothelial, and alveolar cells in mice. Adv. Healthc. Mater. 13, 2400225 (2024).
-
Yan, Y. et al. Branched hydrophobic tails in lipid nanoparticles enhance mRNA delivery for cancer immunotherapy. Biomaterials 301, 122279 (2023).
-
Han, X. et al. In situ combinatorial synthesis of degradable branched lipidoids for systemic delivery of mRNA therapeutics and gene editors. Nat. Commun. 15, 1762 (2024).
-
Knapp, C. M., Guo, P. & Whitehead, K. A. Lipidoid tail structure strongly influences siRNA delivery activity. Cell Mol. Bioeng. 9, 305–314 (2016).
-
Whitehead, K. A. et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat. Commun. 5, 4277 (2014).
-
Shirazi, R. S. et al. Synthesis and characterization of degradable multivalent cationic lipids with disulfide-bond spacers for gene delivery. Biochim. Biophys. Acta Biomembr. 1808, 2156–2166 (2011).
-
Qiu, M. et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc. Natl Acad. Sci. USA 119, e2116271119 (2022).
-
Witzigmann, D. et al. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv. Drug Deliv. Rev. 159, 344–363 (2020).
-
Paunovska, K. et al. Nanoparticles containing oxidized cholesterol deliver mRNA to the liver microenvironment at clinically relevant doses. Adv. Mater. 31, 1807748 (2019).
-
Yang, S. T., Kreutzberger, A. J. B., Lee, J., Kiessling, V. & Tamm, L. K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids 199, 136–143 (2016).
-
Zhang, X., Barraza, K. M. & Beauchamp, J. L. Cholesterol provides nonsacrificial protection of membrane lipids from chemical damage at air–water interface. Proc. Natl Acad. Sci. USA 115, 3255–3260 (2018).
-
Alberts, B. et al. The lipid bilayer. In Molecular Biology of the Cell 4th edn (Garland Science, 2002).
-
Hald Albertsen, C. et al. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 188, 114416 (2022).
-
Briuglia, M. L., Rotella, C., McFarlane, A. & Lamprou, D. A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 5, 231–242 (2015).
-
Patel, S. et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 11, 983 (2020).
-
Kim, J. et al. Engineering lipid nanoparticles for enhanced intracellular delivery of mRNA through inhalation. ACS Nano 16, 14792–14806 (2022).
-
LoPresti, S. T., Arral, M. L., Chaudhary, N. & Whitehead, K. A. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J. Control. Release 345, 819–831 (2022).
-
Semple, S. C. et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28, 172–176 (2010).
-
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020). This article demonstrates that selective organ targeting can alter nanoparticle organ tropism by modulating nanoparticle charge.
-
Du, Z., Munye, M. M., Tagalakis, A. D., Manunta, M. D. I. I. & Hart, S. L. The role of the helper lipid on the DNA transfection efficiency of lipopolyplex formulations. Sci. Rep. 4, 4–9 (2014).
-
Álvarez-Benedicto, E. et al. Optimization of phospholipid chemistry for improved lipid nanoparticle (LNP) delivery of messenger RNA (mRNA). Biomater. Sci. 10, 549–559 (2022).
-
Cheng, X. & Lee, R. J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 99, 129–137 (2016).
-
Koltover, I., Salditt, T., Rädler, J. O. & Safinya, C. R. An inverted hexagonal phase of cationic liposome–DNA complexes related to DNA release and delivery. Science 281, 78–81 (1998).
-
Kauffman, K. J. et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 15, 7300–7306 (2015). This article describes the identification of enduring mRNA–lipid-nanoparticle formulation parameters using factorial design.
-
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
-
Kulkarni, J. A., Witzigmann, D., Leung, J., Tam, Y. Y. C. & Cullis, P. R. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale 11, 21733–21739 (2019).
-
Eygeris, Y., Patel, S., Jozic, A. & Sahay, G. Deconvoluting lipid nanoparticle structure for messenger RNA delivery. Nano Lett. 20, 4543–4549 (2020).
-
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
-
Álvarez-Benedicto, E. et al. Spleen SORT LNP generated in situ CAR T cells extend survival in a mouse model of lymphoreplete B cell lymphoma. Angew. Chem. Int. Edn 62, e202310395 (2023).
-
Safra, T. et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann. Oncol. 11, 1029–1034 (2000).
-
Pozzi, D. et al. Effect of polyethyleneglycol (PEG) chain length on the bio-nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells. Nanoscale 6, 2782–2792 (2014).
-
Suzuki, T. et al. PEG shedding-rate-dependent blood clearance of PEGylated lipid nanoparticles in mice: faster PEG shedding attenuates anti-PEG IgM production. Int. J. Pharm. 588, 119792 (2020).
-
Sanchez, L., Yi, Y. & Yu, Y. Effect of partial PEGylation on particle uptake by macrophages. Nanoscale 9, 288–297 (2017).
-
Nicholas, A. R., Scott, M. J., Kennedy, N. I. & Jones, M. N. Effect of grafted polyethylene glycol (PEG) on the size, encapsulation efficiency and permeability of vesicles. Biochim. Biophys. Acta Biomembr. 1463, 167–178 (2000).
-
Perry, J. L. et al. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 12, 5304–5310 (2012).
-
Jokerst, J. V., Lobovkina, T., Zare, R. N. & Gambhir, S. S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 6, 715–728 (2011).
-
Nosova, A. S. et al. Diversity of PEGylation methods of liposomes and their influence on RNA delivery. MedChemComm 10, 369–377 (2019).
-
Li, M. et al. Brush conformation of polyethylene glycol determines the stealth effect of nanocarriers in the low protein adsorption regime. Nano Lett. 21, 1591–1598 (2021).
-
Tenchov, R., Sasso, J. M. & Zhou, Q. A. PEGylated lipid nanoparticle formulations: immunological safety and efficiency perspective. Bioconj. Chem. 34, 941–960 (2023).
-
Zhou, K. et al. Hydrophobic domain structure of linear-dendritic poly(ethylene glycol) lipids affects RNA delivery of lipid nanoparticles. Mol. Pharm. 17, 1575–1585 (2020).
-
Chen, D., Ganesh, S., Wang, W. & Amiji, M. The role of surface chemistry in serum protein corona-mediated cellular delivery and gene silencing with lipid nanoparticles. Nanoscale 11, 8760–8775 (2019).
-
Berger, M. et al. Effect of PEG anchor and serum on lipid nanoparticles: development of a nanoparticles tracking method. Pharmaceutics 15, 597 (2023).
-
Fenton, O. S. et al. Bioinspired alkenyl amino alcohol ionizable lipid materials for highly potent in vivo mRNA delivery. Adv. Mater. 28, 2939–2943 (2016).
-
Melamed, J. R. et al. Ionizable lipid nanoparticles deliver mRNA to pancreatic β cells via macrophage-mediated gene transfer. Sci. Adv. 9, eade1444 (2023).
-
Hatakeyama, H. et al. Systemic delivery of siRNA to tumors using a lipid nanoparticle containing a tumor-specific cleavable PEG-lipid. Biomaterials 32, 4306–4316 (2011).
-
Ju, Y. et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. ACS Nano 16, 11769–11780 (2022).
-
Dézsi, L. et al. A naturally hypersensitive porcine model may help understand the mechanism of COVID-19 mRNA vaccine-induced rare (pseudo) allergic reactions: complement activation as a possible contributing factor. Geroscience 44, 597–618 (2022).
-
Chaudhary, N. et al. Amine headgroups in ionizable lipids drive immune responses to lipid nanoparticles by binding to the receptors TLR4 and CD1d. Nat. Biomed. Eng. 8, 1483–1498 (2024).
-
Nogueira, S. S. et al. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Appl. Nano Mater. 3, 10634–10645 (2020).
-
Kang, D. D. et al. Engineering LNPs with polysarcosine lipids for mRNA delivery. Bioact. Mater. 37, 86–93 (2024).
-
Luozhong, S. et al. Poly(carboxybetaine) lipids enhance mRNA therapeutics efficacy and reduce their immunogenicity. Nat. Mater. 24, 1852–1861 (2025).
-
Xiao, Y. et al. High-density brush-shaped polymer lipids reduce anti-PEG antibody binding for repeated administration of mRNA therapeutics. Nat. Mater. 24, 1840–1851 (2025).
-
Hinnebusch, A. G., Ivanov, I. P. & Sonenberg, N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 352, 1413–1416 (2016).
-
Gebauer, F. & Hentze, M. W. Molecular mechanisms of translational control. Nat. Rev. Mol. Cell Biol. 5, 827–835 (2004).
-
Mignone, F., Gissi, C., Liuni, S. & Pesole, G. Untranslated regions of mRNAs. Genome Biol. 3, REVIEWS0004 (2002).
-
Karikó, K., Muramatsu, H., Ludwig, J. & Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 39, e142 (2011).
-
Melamed, J. R. et al. Lipid nanoparticle chemistry determines how nucleoside base modifications alter mRNA delivery. J. Control. Release 341, 206–214 (2022).
-
Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005). This article reports that nucleoside-modified RNA suppresses innate immune sensing, laying the foundation for mRNA therapeutics.
-
Granot, Y. & Peer, D. Delivering the right message: challenges and opportunities in lipid nanoparticles-mediated modified mRNA therapeutics — an innate immune system standpoint. Semin. Immunol. 34, 68–77 (2017).
-
Chaudhary, N., Weissman, D. & Whitehead, K. A. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat. Rev. Drug Discov. 20, 817–838 (2021).
-
Karikó, K. & Weissman, D. Naturally occurring nucleoside modifications suppress the immunostimulatory activity of RNA: implication for therapeutic RNA development. Curr. Opin. Drug Discov. Devel. 10, 523–532 (2007).
-
Karikó, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
-
Hajj, K. A. & Whitehead, K. A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2, 17056 (2017).
-
Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019). This article describes lipid H, later known as SM-102, the ionizable lipid in the Moderna COVID-19 mRNA vaccine.
-
Li, Y., Ye, Z., Yang, H. & Xu, Q. Tailoring combinatorial lipid nanoparticles for intracellular delivery of nucleic acids, proteins, and drugs. Acta Pharm. Sin. B 12, 2624–2639 (2022).
-
Ball, R. L., Hajj, K. A., Vizelman, J., Bajaj, P. & Whitehead, K. A. Lipid nanoparticle formulations for enhanced co-delivery of siRNA and mRNA. Nano Lett. 18, 3814–3822 (2018).
-
Fletcher, S. et al. In vivo studies of dialkynoyl analogues of DOTAP demonstrate improved gene transfer efficiency of cationic liposomes in mouse lung. J. Med. Chem. 49, 349–357 (2006).
-
Ngo, W. et al. Identifying cell receptors for the nanoparticle protein corona using genome screens. Nat. Chem. Biol. 18, 1023–1031 (2022).
-
Suzuki, Y. et al. Splenic B cell-targeting lipid nanoparticles for safe and effective mRNA vaccine delivery. J. Control. Release 382, 113687 (2025).
-
Voke, E. et al. Protein corona formed on lipid nanoparticles compromises delivery efficiency of mRNA cargo. Nat. Commun. 16, 8699 (2025).
-
Hardianto, A., Muscifa, Z. S., Widayat, W., Yusuf, M. & Subroto, T. The effect of ethanol on lipid nanoparticle stabilization from a molecular dynamics simulation perspective. Molecules 28, 4836 (2023).
-
Henderson, M. I., Eygeris, Y., Jozic, A., Herrera, M. & Sahay, G. Leveraging biological buffers for efficient messenger RNA delivery via lipid nanoparticles. Mol. Pharm. 19, 4275–4285 (2022).
-
Cheng, M. H. Y. et al. Induction of bleb structures in lipid nanoparticle formulations of mRNA leads to improved transfection potency. Adv. Mater. 35, 2303370 (2023).
-
Lehman, S. E. et al. Particle metrology approach to understanding how storage conditions affect long-term liposome stability. Langmuir 39, 12313–12323 (2023).
-
Love, K. T. et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA 107, 1864–1869 (2010).
-
Zhang, H. et al. Together is better: mRNA co-encapsulation in lipoplexes is required to obtain ratiometric co-delivery and protein expression on the single cell level. Adv. Sci. 9, 2102072 (2022).
-
Miller, J. B. et al. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew. Chem. Int. Edn 56, 1059–1063 (2017).
-
Han, J. P. et al. In vivo delivery of CRISPR–Cas9 using lipid nanoparticles enables antithrombin gene editing for sustainable hemophilia A and B therapy. Sci. Adv. 8, 6901 (2022).
-
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 20, 701–710 (2021).
-
Fessi, H., Puisieux, F., Devissaguet, J. P., Ammoury, N. & Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 55, R1–R4 (1989).
-
Liu, Y. et al. Formulation of nanoparticles using mixing-induced nanoprecipitation for drug delivery. Ind. Eng. Chem. Res. 59, 4134–4149 (2020).
-
Martínez Rivas, C. J. et al. Nanoprecipitation process: from encapsulation to drug delivery. Int. J. Pharm. 532, 66–81 (2017).
-
Miladi, K. et al. Nanoprecipitation process: from particle preparation to in vivo applications. Polym. Nanopart. Nanomed. 445, 17–53 (2016).
-
Johnson, B. K. & Prud’homme, R. K. Chemical processing and micromixing in confined impinging jets. AIChE J. 49, 2264–2282 (2003).
-
Maeki, M. et al. Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers. PLoS ONE 12, e0187962 (2017).
-
Nong, J. et al. Multi-stage-mixing to control the supramolecular structure of lipid nanoparticles, thereby creating a core-then-shell arrangement that improves performance by orders of magnitude. Preprint at bioRxiv https://doi.org/10.1101/2024.11.12.623321 (2024).
-
Strelkova Petersen, D. M., Chaudhary, N., Arral, M. L., Weiss, R. M. & Whitehead, K. A. The mixing method used to formulate lipid nanoparticles affects mRNA delivery efficacy and organ tropism. Eur. J. Pharm. Biopharm. 192, 126–135 (2023).
-
Hajj, K. A. et al. Branched-tail lipid nanoparticles potently deliver mRNA in vivo due to enhanced ionization at endosomal pH. Small 15, 1805097 (2019).
-
Bhattacharjee, S. DLS and zeta potential — what they are and what they are not? J. Control. Release 235, 337–351 (2016).
-
Malburet, C. et al. Size and charge characterization of lipid nanoparticles for mRNA vaccines. Anal. Chem. 94, 4677–4685 (2022).
-
Loughney, J. W., Minsker, K., Ha, S. & Rustandi, R. R. Development of an imaged capillary isoelectric focusing method for characterizing the surface charge of mRNA lipid nanoparticle vaccines. Electrophoresis 40, 2602–2609 (2019).
-
Ryals, R. C. et al. The effects of PEGylation on LNP based mRNA delivery to the eye. PLoS ONE 15, e0241006 (2020).
-
Suk, J. S., Xu, Q., Kim, N., Hanes, J. & Ensign, L. M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 99, 28–51 (2016).
-
Chen, S. et al. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Control. Release 235, 236–244 (2016).
-
Lam, K. et al. Optimizing lipid nanoparticles for delivery in primates. Adv. Mater. 35, 2211420 (2023).
-
Hassett, K. J. et al. Impact of lipid nanoparticle size on mRNA vaccine immunogenicity. J. Control. Release 335, 237–246 (2021).
-
Zhang, J., Fan, H., Levorse, D. A. & Crocker, L. S. Ionization behavior of amino lipids for siRNA delivery: determination of ionization constants, SAR, and the impact of lipid pKa on cationic lipid–biomembrane interactions. Langmuir 27, 1907–1914 (2011).
-
Guimaraes, P. P. G. et al. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J. Control. Release 316, 404–417 (2019).
-
McClure, W. O. & Edelman, G. M. Fluorescent probes for conformational states of proteins. I. Mechanism of fluorescence of 2-p-toluidinylnaphthalene-6-sulfonate, a hydrophobic probe. Biochemistry 5, 1908–1919 (1966).
-
Koh, C. G. et al. Delivery of antisense oligodeoxyribonucleotide lipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing. J. Control. Release 141, 62–69 (2010).
-
Zhigaltsev, I. V. et al. Bottom-up design and synthesis of limit size lipid nanoparticle systems with aqueous and triglyceride cores using millisecond microfluidic mixing. Langmuir 28, 3633–3640 (2012).
-
Sebastiani, F. et al. Apolipoprotein E binding drives structural and compositional rearrangement of mRNA-containing lipid nanoparticles. ACS Nano 15, 6709–6722 (2021).
-
Miao, L. et al. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 11, 2424 (2020).
-
Trollmann, M. F. W. & Böckmann, R. A. mRNA lipid nanoparticle phase transition. Biophys. J. 121, 3927–3939 (2022).
-
Kim, B. et al. Optimization of storage conditions for lipid nanoparticle-formulated self-replicating RNA vaccines. J. Control. Release 353, 241–253 (2023).
-
Ball, R. L., Bajaj, P. & Whitehead, K. A. Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 12, 305–315 (2016).
-
Zhao, P. et al. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 5, 358–363 (2020).
-
Suzuki, Y. et al. Design and lyophilization of lipid nanoparticles for mRNA vaccine and its robust immune response in mice and nonhuman primates. Mol. Ther. Nucleic Acids 30, 226–240 (2022).
-
Li, M. et al. Lyophilization process optimization and molecular dynamics simulation of mRNA-LNPs for SARS-CoV-2 vaccine. npj Vaccines 8, 153 (2023).
-
Guo, J. et al. Nucleic acid delivery by lipid nanoparticles for organ targeting. Chin. Chem. Lett. 36, 110849 (2025).
-
Ma, Y., Li, S., Lin, X. & Chen, Y. A perspective of lipid nanoparticles for RNA delivery. Exploration 4, 20230147 (2024).
-
Besin, G. et al. Accelerated blood clearance of lipid nanoparticles entails a biphasic humoral response of B-1 followed by B-2 lymphocytes to distinct antigenic moieties. Immunohorizons 3, 282–293 (2019).
-
Sanchez, A. J. D. S. et al. Substituting poly(ethylene glycol) lipids with poly(2-ethyl-2-oxazoline) lipids improves lipid nanoparticle repeat dosing. Adv. Healthc. Mater. 13, 2304033 (2024).
-
Kedmi, R., Ben-Arie, N. & Peer, D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials 31, 6867–6875 (2010).
-
Kvietys, P. R. in The Gastrointestinal Circulation Ch. 6 (Morgan & Claypool Life Sciences, 2010).
-
Kanasty, R. L., Whitehead, K. A., Vegas, A. J. & Anderson, D. G. Action and reaction: the biological response to siRNA and its delivery vehicles. Mol. Ther. 20, 513–524 (2012).
-
Jacobs, F., Wisse, E. & De Geest, B. The role of liver sinusoidal cells in hepatocyte-directed gene transfer. Am. J. Pathol. 176, 14–21 (2010).
-
Tanuma, Y., Ohata, M., Ito, T. & Uchida, K. Electron microscopic studies on the sinusoidal cells in the monkey liver. Arch. Histol. Jpn. 46, 401–426 (1983).
-
Mosquera, J., García, I. & Liz-Marzán, L. M. Cellular uptake of nanoparticles versus small molecules: a matter of size. Acc. Chem. Res. 51, 2305–2313 (2018).
-
Gilleron, J. et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 31, 638–646 (2013). This article reports the quantification of intracellular lipid nanoparticle trafficking, showing that only about 1–2% achieve endosomal escape.
-
Hunter, M. R. et al. Understanding intracellular biology to improve mRNA delivery by lipid nanoparticles. Small Methods 7, 2201695 (2023).
-
Mo, Y. et al. Lipid–siRNA organization modulates the intracellular dynamics of lipid nanoparticles. J. Am. Chem. Soc. 147, 10430–10445 (2025).
-
Sahay, G. et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 31, 653–658 (2013).
-
Iwakawa, K. et al. Cubic phase-inducible zwitterionic phospholipids improve the functional delivery of mRNA. Adv. Sci. 12, 2413016 (2025).
-
Mochizuki, S. et al. The role of the helper lipid dioleoylphosphatidylethanolamine (DOPE) for DNA transfection cooperating with a cationic lipid bearing ethylenediamine. Biochim. Biophys. Acta Biomembr. 1828, 412–418 (2013).
-
Zheng, L., Bandara, S. R., Tan, Z. & Leal, C. Lipid nanoparticle topology regulates endosomal escape and delivery of RNA to the cytoplasm. Proc. Natl Acad. Sci. USA 120, e2301067120 (2023).
-
Gindy, M. E. et al. Mechanism of macromolecular structure evolution in self-assembled lipid nanoparticles for siRNA delivery. Langmuir 30, 4613–4622 (2014).
-
Tesei, G. et al. Lipid shape and packing are key for optimal design of pH-sensitive mRNA lipid nanoparticles. Proc. Natl Acad. Sci. USA 121, e2311700120 (2024).
-
Herrera, M. et al. Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery. Biomater. Sci. 9, 4289–4300 (2021).
-
Pardi, N. et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 217, 345–351 (2015). This article is one of the first to report mRNA delivery by lipid nanoparticles.
-
Wang, C. et al. Blood–brain-barrier-crossing lipid nanoparticles for mRNA delivery to the central nervous system. Nat. Mater. 24, 1653–1663 (2025).
-
Kuzminich, Y. et al. Lipid nanoparticles deliver mRNA to the blood–brain barrier. Nano Res. 17, 9126–9134 (2024).
-
Tiwade, P. B., Ma, Y., VanKeulen-Miller, R. & Fenton, O. S. A lung-expressing mRNA delivery platform with tunable activity in hypoxic environments. J. Am. Chem. Soc. 146, 17365–17376 (2024).
-
Tiwade, P. B. et al. Customizable polymeric nanoparticle materials optimized on hypoxic cells facilitate mRNA expression in the lungs in vivo. Adv. Healthc. Mater. 14, 2500245 (2025).
-
Omo-Lamai, S. et al. Physicochemical targeting of lipid nanoparticles to the lungs induces clotting: mechanisms and solutions. Adv. Mater. 36, 2312026 (2024).
-
Turnbull, I. C. et al. Myocardial delivery of lipidoid nanoparticle carrying modRNA induces rapid and transient expression. Mol. Ther. 24, 66–75 (2016).
-
Isaac, I. et al. Reengineering endogenous targeting lipid nanoparticles (ENDO) for systemic delivery of mRNA to pancreas. Adv. Mater. 37, 2507657 (2025).
-
Sago, C. D. et al. Nanoparticles that deliver RNA to bone marrow identified by in vivo directed evolution. J. Am. Chem. Soc. 140, 17095–17105 (2018).
-
Lian, X. et al. Bone-marrow-homing lipid nanoparticles for genome editing in diseased and malignant haematopoietic stem cells. Nat. Nanotechnol. 19, 1409–1417 (2024).
-
Kim, H. et al. Lipid nanoparticle-mediated mRNA delivery to CD34+ cells in rhesus monkeys. Nat. Biotechnol. 43, 1813–1820 (2025).
-
Chaudhary, N. et al. Lipid nanoparticle structure and delivery route during pregnancy dictate mRNA potency, immunogenicity, and maternal and fetal outcomes. Proc. Natl Acad. Sci. USA 121, e2307810121 (2024).
-
Swingle, K. L. et al. Placenta-tropic VEGF mRNA lipid nanoparticles ameliorate murine pre-eclampsia. Nature 638, 412–421 (2025).
-
Hofbauer, S. I. et al. Cytokine mRNA delivery and local immunomodulation in the placenta using lipid nanoparticles. Adv. Ther. 8, e00148 (2025).
-
Lee, E. et al. Elasticity-driven nanomechanical interaction to improve the targeting ability of lipid nanoparticles in the malignant tumor microenvironment. Adv. Sci. 12, 2502073 (2025).
-
Riley, R. S. et al. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 7, 1028–1041 (2021).
-
Palanki, R. et al. In utero delivery of targeted ionizable lipid nanoparticles facilitates in vivo gene editing of hematopoietic stem cells. Proc. Natl Acad. Sci. USA 121, e2400783121 (2024).
-
Liu, C., Jiang, X., Gan, Y. & Yu, M. Engineering nanoparticles to overcome the mucus barrier for drug delivery: design, evaluation and state-of-the-art. Med. Drug Discov. 12, 100110 (2021).
-
Kulkarni, J. A., Witzigmann, D., Chen, S., Cullis, P. R. & Van Der Meel, R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 52, 2435–2444 (2019).
-
Benet, L. Z. & Zia-Amirhosseini, P. Basic principles of pharmacokinetics. Toxicol. Pathol. 23, 115–123 (1995).
-
Sedic, M. et al. Safety evaluation of lipid nanoparticle-formulated modified mRNA in the Sprague–Dawley rat and cynomolgus monkey. Vet. Pathol. 55, 341–354 (2018).
-
August, A. et al. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against chikungunya virus. Nat. Med. 27, 2224–2233 (2021).
-
Maier, M. A. et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 21, 1570–1578 (2013).
-
Ndeupen, S. et al. The mRNA–LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 24, 103479 (2021).
-
Gao, S. et al. Improving adenine base editing precision by enlarging the recognition domain of CRISPR–Cas9. Nat. Commun. 16, 2081 (2025).
-
Khirallah, J. et al. In vivo base editing of Angptl3 via lipid nanoparticles to treat cardiovascular disease. Mol. Ther. Nucleic Acids 36, 102486 (2025).
-
Perez-Garcia, C. G. et al. Development of an mRNA replacement therapy for phenylketonuria. Mol. Ther. Nucleic Acids 28, 87–98 (2022).
-
Wei, T. et al. Lung SORT LNPs enable precise homology-directed repair mediated CRISPR/Cas genome correction in cystic fibrosis models. Nat. Commun. 14, 7322 (2023).
-
Whitehead, K. A., Langer, R. & Anderson, D. G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 8, 129–138 (2009).
-
Young, T. L. et al. Clinical delivery of circular RNA: lessons learned from RNA drug development. Adv. Drug Deliv. Rev. 197, 114826 (2023).
-
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
-
Billingsley, M. M. et al. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 20, 1578–1589 (2020).
-
Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021).
-
Cohn, D. M. et al. CRISPR-based therapy for hereditary angioedema. N. Engl. J. Med. 392, 458–467 (2025).
-
Truong, B. et al. Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc. Natl Acad. Sci. USA 116, 21150–21159 (2019).
-
Rake, J. et al. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease type I (ESGSD I). Eur. J. Pediat. 161, S20–S34 (2002).
-
Head, P. S. E., Meier, J. L. & Venditti, C. P. New insights into the pathophysiology of methylmalonic acidemia. J. Inherit. Metab. Dis. 46, 436–449 (2023).
-
Perrie, Y. et al. The impact of ageing on the barriers to drug delivery. J. Control. Release 161, 389–398 (2012).
-
Poley, M. et al. Sex-based differences in the biodistribution of nanoparticles and their effect on hormonal, immune, and metabolic function. Adv. Nanobiomed. Res. 2, 2200089 (2022).
-
Bae, S. H. et al. Rational design of lipid nanoparticles for enhanced mRNA vaccine delivery via machine learning. Small 21, 2405618 (2025).
-
Wang, W. et al. Artificial intelligence-driven rational design of ionizable lipids for mRNA delivery. Nat. Commun. 15, 10804 (2024).
-
Müller, R. H., Mäder, K. & Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery — a review of the state of the art. Eur. J. Pharm. Biopharm. 50, 161–177 (2000).
-
Szoka, F. & Papahadjopoulos, D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu. Rev. Biophys. Bioeng. 9, 467–508 (1980).
-
Eldem, T., Speiser, P. & Hincal, A. Optimization of spray-dried and -congealed lipid micropellets and characterization of their surface morphology by scanning electron microscopy. Pharm. Res. 8, 47–54 (1991).
-
Wesselhoeft, R. A., Kowalski, P. S. & Anderson, D. G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9, 2629 (2018).
-
Grimm, D. & Kay, M. A. Therapeutic short hairpin RNA expression in the liver: viral targets and vectors. Gene Ther. 13, 563–575 (2006).
-
Diener, C., Keller, A. & Meese, E. Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet. 38, 613–626 (2022).
-
Jain, R. et al. MicroRNAs enable mRNA therapeutics to selectively program cancer cells to self-destruct. Nucleic Acid Ther. 28, 285–296 (2018).
-
Mahata, B., Mukherjee, S., Mishra, S., Bandyopadhyay, A. & Adhya, S. Functional delivery of a cytosolic tRNA into mutant mitochondria of human cells. Science 314, 471–474 (2006).
-
Albers, S. et al. Engineered tRNAs suppress nonsense mutations in cells and in vivo. Nature 618, 842–848 (2023).
-
Keefe, A. D., Pai, S. & Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 9, 537–550 (2010).
-
Torres-Vázquez, B. et al. In vitro selection of high affinity DNA and RNA aptamers that detect hepatitis C virus core protein of genotypes 1 to 4 and inhibit virus production in cell culture. J. Mol. Biol. 434, 167501 (2022).