From axolotls to zebrafish, stem cells possess remarkable regenerative abilities, enabling them to restore lost limbs and repair damaged organs. These extraordinary capabilities, however, are not fully mirrored in humans. Despite the significant potential of human stem cells for treating a wide range of conditions, the field is still developing, especially as more physiologically relevant stem cell assays emerge.
Human stem cells are broadly classified into three principal categories: embryonic stem cells (ESCs), adult stem cells (ASCs), and induced pluripotent stem cells (iPSCs). ESCs are pluripotent cells derived from early-stage embryos that have the capacity to differentiate into nearly any cell type. ASCs, in contrast, are multipotent and primarily differentiate primarily into the cell types of their tissue of origin. Human iPSCs represent a significant scientific breakthrough, whereby somatic cells such as those from skin or blood can be reprogrammed to a pluripotent state through the introduction of specific transcription factors and conditions.
The International Society for Stem Cell Research (ISSCR) recently convened a symposium emphasizing neural cell applications. GEN spoke to several of the presenters on how they are utilizing their technologies to advance the field.
Guide-integrated adult stem cells
To utilize stem cells for regenerative medicine, they must not only be safe but also maintain their plasticity to differentiate into tissue-specific cell types, according to Min Hu, MD, PhD, founder, CEO, and president of APstem Therapeutics. She elaborates, “Although traditional pluripotent stem cells can differentiate into a variety of cells, there is a risk of tumorigenesis, while terminally differentiated cells such as mesenchymal stromal cells (MSCs) lack the ability to rebuild complex tissues. To overcome such stem cell therapy limitations, we developed a novel stem cell type, guide-integrated adult stem cells (giaSCs).”

Founder, CEO
APstem Therapeutics
Hu says the giaSCs are derived from the interaction of mesenchymal stromal cells (MSCs) mixed with a group of blood stem cells. She explains, “The giaSCs are a newly discovered cell derived from adult blood and have a completely different gene profile from other stem cell types including the cells they are derived from, that is, they are neither guide cells nor MSCs. Further, these giaSCs can differentiate into all three germ layer cell types, do not form tumors, and do not invoke an immune response.”
In preclinical studies, scientists utilized giaSCs for the treatment of mouse models of skin wounds and small intestinal tissue damage. For the skin tissue reconstitution assay, the giaSC were mixed with a fibrin gel and transplanted into the wounds. As compared to controls, the treatment accelerated wound closure and caused full regeneration of the skin structures, including hair follicles. For the model of intestinal damage, following systemic administration of the cells, a variety of assessments were performed. The scientists determined not only that the giaSC had differentiated into enterocytes but also had participated in the repair of the damaged area. Interestingly, transplantation into mice without tissue damage had no effect on normal tissue maintenance.
Hu emphasizes that giaSCs have low immunogenicity, permitting potential use for “off-the-shelf” allogeneic transplantation. “Also critical is that, unlike other stem cells, they are not tumorigenic or toxic. They are safe, effective, and scalable.”
The company is initially focusing studies on diabetes applications, including wound healing. Since giaSCs can be administered topically, subcutaneously, or intravenously, they are also are working on neurodegeneration and aging-related diseases. Hu laments, “We are continuing investigations, but the number one challenge, as a small company, is raising funds to continue our therapeutic effort!”
iNeuron model systems
Stem cells differentiated into functional neurons can be used in assays for in vitro modeling of neurodegenerative conditions as well as tools for developing therapeutics.

Principal Scientist Group Leader
Genentech
Maheswara Reddy Emani, PhD, principal scientist and group leader at Genentech, reports how their scientists are employing a specialized type of stem cell-derived neuron. “iPSC-derived NGN2-induced neurons (or iNeurons) are differentiated from human iPSCs with transcription factor Neurogenin-2 (NGN2) to rapidly become neurons. This method allows us to efficiently produce large quantities of human neurons in a scalable and reproducible way, making them ideal for high-throughput screening of compound libraries.”
The iNeurons are also being utilized as models of chemical toxicity and mechanical damage (axotomy). Emani continues, “These assays simulate conditions of neuronal injury, allowing for the study of mechanisms of neuroprotection and axon regeneration in vitro. By integrating automated imaging and AI analysis methods, we can efficiently measure axon degeneration and regeneration, providing robust data with reduced manual efforts.”
Emani emphasizes that studying neuroprotection and axon regeneration is valuable because it tackles the core mechanisms of neuronal survival and repair that are disrupted. “Neuroprotection focuses on preserving neurons and preventing further damage following injury or disease, while axon regeneration aims to restore connectivity by promoting the regrowth of damaged axons. Together, these processes are essential for maintaining or recovering neural function. Research in this area supports the discovery and development of therapies for neurodegenerative diseases as well as for traumatic injuries like spinal cord damage.”

According to Emani, Genentech’s human-relevant neuronal model systems are designed to accelerate the discovery and validation of therapies for neurological conditions. “Our goal is to translate cutting-edge science into effective treatments that address the underlying mechanisms of neurodegeneration.”
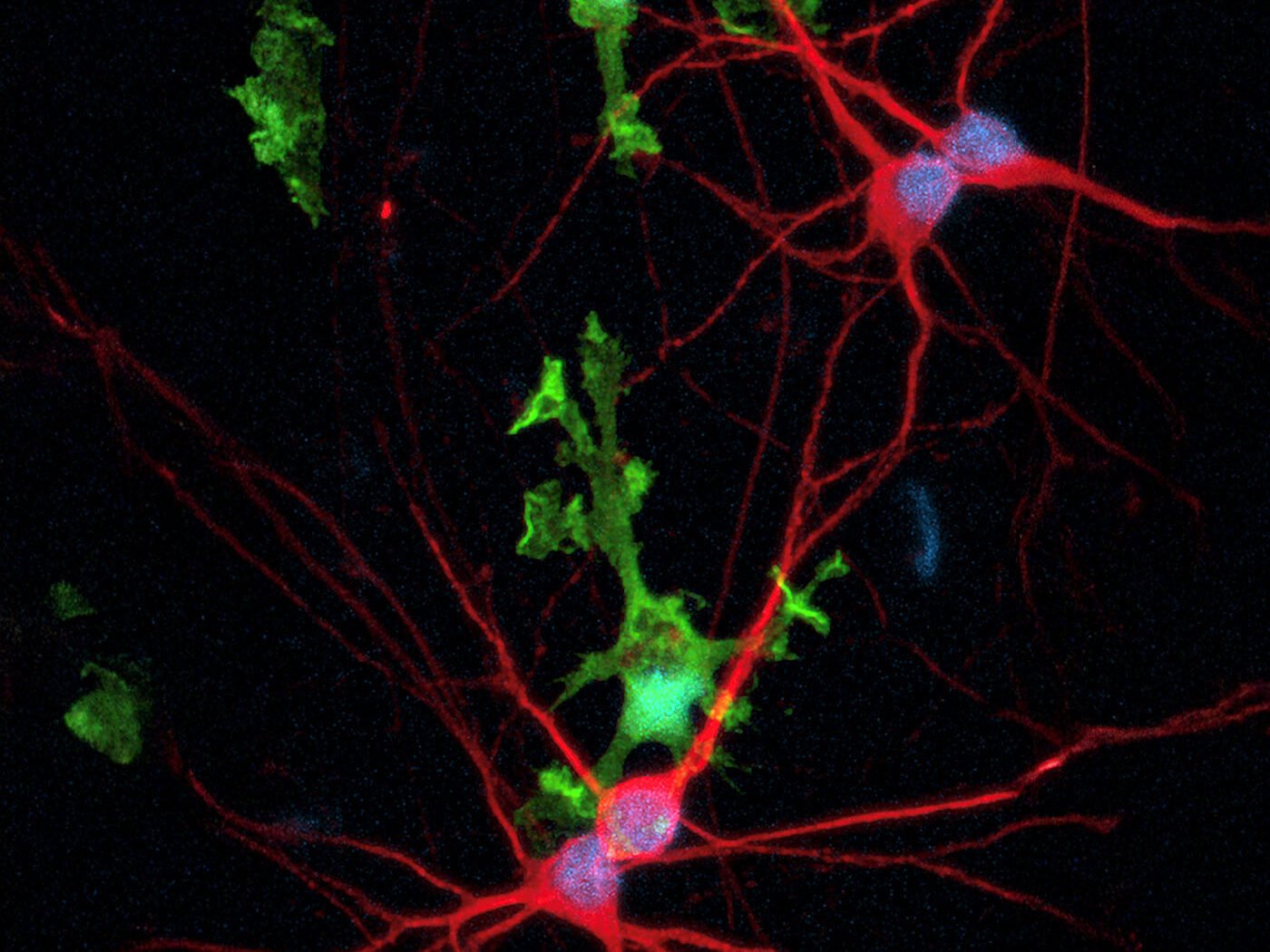
Microglia toolbox
“Microglia, the resident macrophages of the brain, are essential for neural homeostasis,” points out Rebecca Northeast, PhD, senior project manager at bit.bio. She continues, “They are a fast-growing target for therapeutics as they regulate neurogenesis, synaptic remodeling, and act as first responders to injury or infection. Dysregulated microglial function is implicated in many neurodegenerative diseases such as Alzheimer’s disease.”

Senior Project Manager, bit.bio
Northeast says, while most cellular and animal microglial models fail to recapitulate the human system, “Our human iPSC-derived microglia offer greater physiological relevance to human biology. They are especially useful for supporting early-stage drug discovery with relevant readouts from functional and phenotypic assays such as phagocytosis and transcriptomic profiling.”
The company creates “ioMicroglia” cells (and a host of other induced cell types) utilizing their opti-ox cell-programming technology. Northeast explains, “As opti-ox is an inducible gene expression system, when it is integrated into an iPSC we can amplify the iPSCs to billions of cells, then switch on opti-ox, which then triggers the expression of a unique combination of transcription factors for a period of time. Within days, the whole population of iPSCs convert into the cell type of interest, in this case, microglia. Being able to culture consistent human microglia from a frozen vial and have them ready for assays in just 10 days is a game-changer compared to what is currently available to researchers.”
cell-programming technology. Northeast explains, “As opti-ox is an inducible gene expression system, when it is integrated into an iPSC we can amplify the iPSCs to billions of cells, then switch on opti-ox, which then triggers the expression of a unique combination of transcription factors for a period of time. Within days, the whole population of iPSCs convert into the cell type of interest, in this case, microglia. Being able to culture consistent human microglia from a frozen vial and have them ready for assays in just 10 days is a game-changer compared to what is currently available to researchers.”
The ioMicroglia can be utilized in assays such as for disease modeling, CRISPR screens, and neurodegeneration drug discovery. Northeast elaborates, “We engineered Alzheimer’s disease-relevant genetic mutations in our wild-type ioMicroglia. This enables researchers to directly study the effects of a single mutation, unlike comparisons from patient donor-derived cells.”
For CRISPR screens, Northeast says the company developed “CRISPRko-Ready ioMicroglia, which constitutively express Cas9, enabling high-throughput CRISPR screening and reducing workflow duration from months to days.”
According to Northeast, the company also provides a range of products for neurodegeneration drug discovery. “Multicellular 2D and 3D in vitro models are becoming increasingly popular among scientists to better represent the human brain, as such, we developed microglia constitutively expressing green fluorescent protein (GFP) for easy cell visualization in these complex systems.”
Differentiation versatility and more
To jumpstart their stem cell research, some investigators may turn to commercially available, well-characterized stem cells and products. Erin Knock, PhD, associate director of neural biology at STEMCELL Technologies, comments, “For example, our SCTi003-A is a healthy control human iPSC line derived from the peripheral blood mononuclear cells of a 48-year-old Caucasian female of European ancestry. It’s ethically sourced using Institutional Review Board protocols, with informed consent for both academic and commercial research use only.”
According to Knock, the cell line is valuable for many types of applications. “The SCTi003-A line can be used to generate cell types from all three embryonic germ layers, with 30 cell types verified to date, including neural progenitor cells, different neuron subtypes, astrocytes, microglia, cardiomyocytes, and hematopoietic progenitor cells. It can also be used to generate complex 3D models, including intestinal organoids and neural organoids. There are now over 20 peer-reviewed publications using this line, demonstrating its differentiation capabilities and applications.”

For researchers wanting to bypass lengthy differentiation protocols, the company also provides differentiated cell types, including retinal pigment epithelial cells, neural progenitors, and neuron precursors generated from SCTi003-A. Knock elaborates, “For example, to generate specific neural cell types, we use STEMdiff differentiation media, such as the forebrain neuron, microglia, and astrocyte kits, along with their respective maturation kits. These media are formulated to mimic in vivo signaling environments through the timed application of small molecules and growth factors. For characterization, we recommend assays like immunocytochemistry, electrophysiology, and gene expression profiling to our customers.”
differentiation media, such as the forebrain neuron, microglia, and astrocyte kits, along with their respective maturation kits. These media are formulated to mimic in vivo signaling environments through the timed application of small molecules and growth factors. For characterization, we recommend assays like immunocytochemistry, electrophysiology, and gene expression profiling to our customers.”
Knock indicates that the company’s human iPSC-derived cells support many applications such as drug discovery, toxicity testing, and disease modeling, as well as serve as consistent controls in custom workflows.
Upcoming launches include human iPSC-derived astrocytes, endothelial cells, and genome-edited lines tailored for targeted disease modeling applications. Knock shares, “Beyond product development, we’re also focused on empowering researchers through training and community-driven resources. That’s why we’ve partnered with the ISSCR to launch a virtual course built around their quality standards. This course was announced at ISSCR 2025 and will be made available in the coming months.”
The post Designer Stem Cells Power New R&D Assay Systems appeared first on GEN – Genetic Engineering and Biotechnology News.