Academic institutes are shaping the future of personalized therapeutics through expanded in-house cGMP manufacturing facilities and increasingly tight integrations with industry. This degree of alignment helps drug substances move from ideation to clinical trials, to market approval applications, without ever leaving the institute.
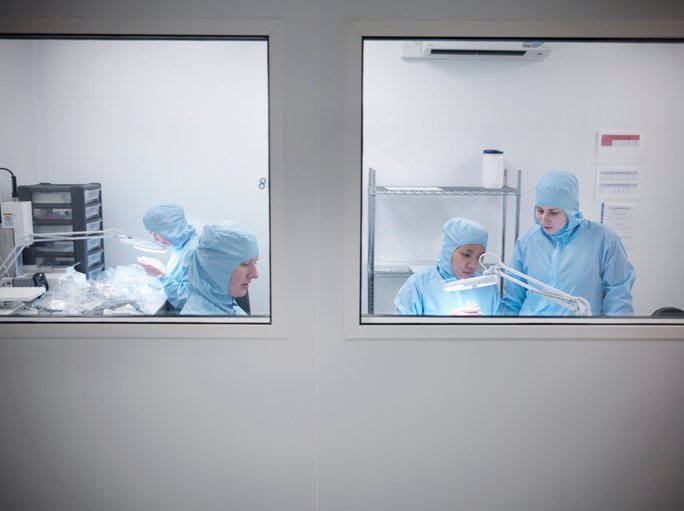
Roswell Park Comprehensive Cancer Center (CCC) is a case in point. Located in Buffalo, NY, it is one of the first three cancer centers to be designated (in 1972) as a comprehensive cancer center by the National Cancer Institute. A few months ago, it expanded its good manufacturing practice (GMP) engineering and cell manufacturing (GEM) facility by 11,000 square feet. That makes it the largest academic GMP facility in the United States.
“We have the largest clean room facility—by room number—in the country,” Chris Choi, PhD, senior vice president of industry partnership and technical director of the GMP facility, tells GEN. That’s 20 clean rooms, compared to the three to six he says most academic GMP facilities offer. The space breaks down to roughly 9,000 square feet (14 rooms) for cell manufacturing; about 1,500 square feet for viral vector production; and approximately 600 square feet for plasmid production.
The GEM facility enables Roswell Park to produce sufficient cell therapies to accommodate several hundred patients per year, running a single shift. “Operationally, we can support the treatment or manufacture of thousands of products for patients per year,” Choi says. The center currently has eight clinical trials underway.
Industry expertise
Therapeutic production capacity is important, but that’s not what propels CGT institutes forward. “Most academic institutes can’t really translate ideas from the benchtop into the clinic,” Choi admits. Therefore, leading academic institutes are also recruiting experts from industry, not just from other academic institutions.
For example, before joining Roswell CCC, Choi was at CRISPR Therapeutics and KITE, where he was involved with investigational new drugs, commercial biologics license agreements (BLAs), and marketing authorization applications (MAAs). Now, forward-thinking CGT institutes are actively seeking people like Choi, who have expertise in development and commercialization, as well as science.
As academic institutes become increasingly aligned with industry, they also become better equipped to help their partners take ideas not just from benchtop to bedside, but through the regulatory process to market authorization, using wholly in-house capabilities. That, Choi says, includes having “the right processes, documentation, and infrastructure for eventual advancement to BLAs or MAAs.”
The other benefit of today’s more capable academic institutes, and Roswell Park CCC in particular, Choi adds, is that “when we build things in-house, we can do it faster, have more control, and help the patients we have here as soon as the products are taken into the clinic.” And that’s good for everyone.
The post Expanding Expertise and Capabilities Propel CGT Institutes appeared first on GEN – Genetic Engineering and Biotechnology News.