Researchers headed by a team at the The Barcelona Institute of Science and Technology (BIST) Centre for Genomic Regulation (CRG) have discovered a protein, eukaryotic translation initiation factor 2A (eIF2A) that appears to be critical for steering melanoma cancer cells as they spread throughout the body. eIF2A is generally thought to spring into action when a cell is under stress, helping ribosomes launch protein synthesis. The newly reported laboratory study suggests that eIF2A has a completely different role in melanoma, helping cancerous cells control movement. The results indicate that the malignant cells become dependent on this protein to migrate, pointing to new strategies for obstructing metastasis.
“In this field, many potential therapeutic targets prove either redundant or essential to normal cells, but the discovery of a protein that quietly makes itself indispensable only when cells become metastatic could be a rare catch,” said CRG researcher Fátima Gebauer, PhD. “Any potential vulnerability counts…Targeting eIF2A could be a new strategy to impede melanoma breaking free and seeding tumors elsewhere.”
Gebauer is lead and corresponding author of the team’s published paper in Science Advances (“eIF2A regulates cell migration in a translation-independent manner,”) in which they stated, “Here, we uncover a function of the alternative translation initiation factor eIF2A in promoting migration by enforcing centrosome composition and orientation.”
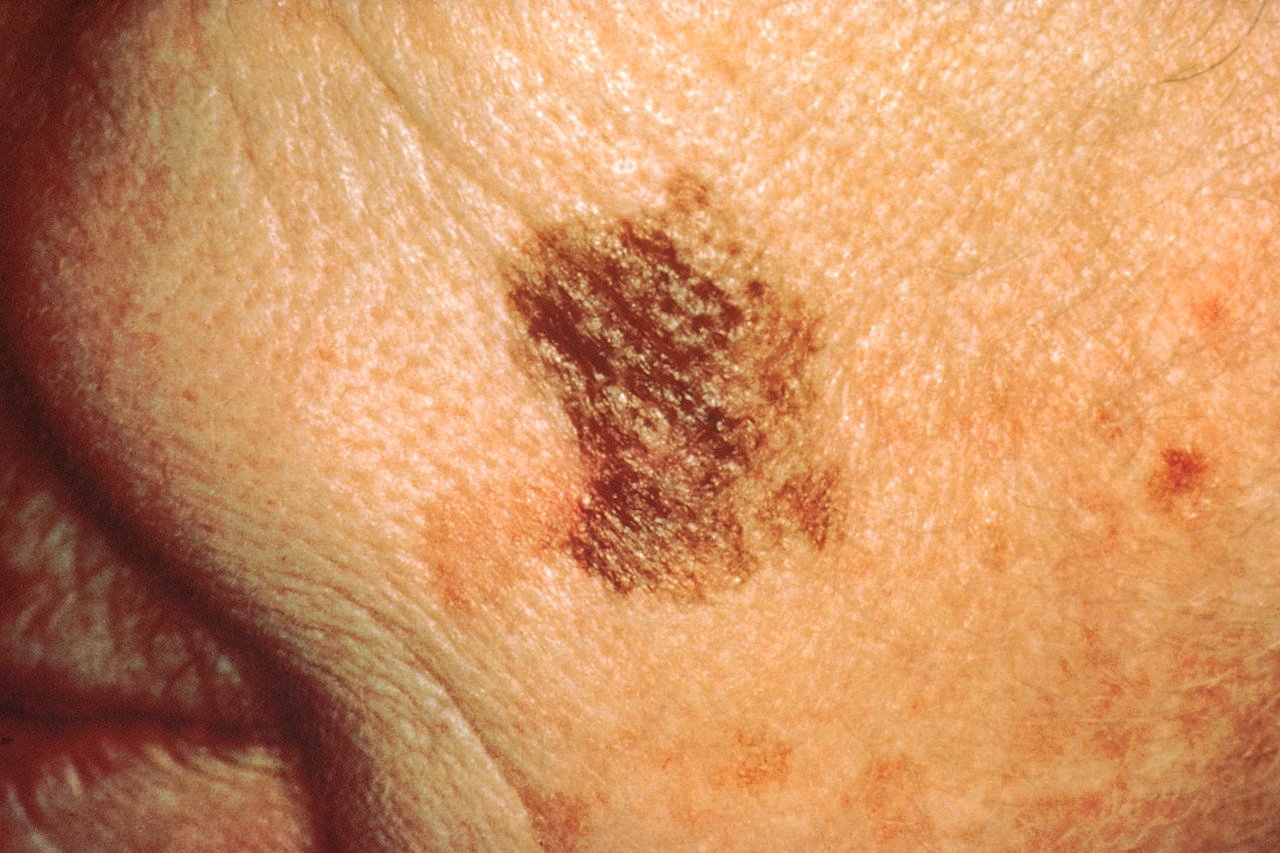
Despite accounting for only a fraction of skin cancer cases, melanoma kills almost 60,000 people worldwide each year. The five-year survival rate for localized melanoma is around 99%, while it is significantly lower for metastatic melanoma, around 35% for distant spread. Understanding how malignant cells metastasize is crucial to improve healthcare outcomes.
![The image shows eIF2A at the centrosome (white) [Jennifer Jungfleisch/Centro de Regulación Genómica]](https://www.genengnews.com/wp-content/uploads/2025/08/low-res-9-300x300.jpeg)
Protein synthesis is a fundamental cellular process that allows cells to fine-tune their responses and adapt to various stimuli and environmental changes, the authors wrote. “This capability is particularly crucial in the context of cancer, as cancer cells need to modulate protein synthesis to drive their aggressive behavior and survival under diverse conditions.” Translation initiation is one of the most highly regulated steps in protein synthesis, and while canonical translation initiation relying on the protein eIF2α has been the focus of research, more recent studies have begun to uncover a growing importance for alternative initiation mechanisms in cancer, the team pointed out. “One such pathway involves the alternative initiation factor 2A (eIF2A),” they stated. “eIF2A has been mainly implicated in translation initiation under stress conditions where the canonical factor eIF2α is inactivated by phosphorylation, and eIF2A takes over in binding the initiator tRNA and promoting initiation codon recognition.”
Melanoma is characterized by its high metastatic potential and resistance to conventional therapies, the researchers continued. Metastatic melanoma is aggressive, and has a poor prognosis, presenting “…an intriguing context for investigating the potential functions of eIF2A.”
For their reported study, and working with a pair of matched human skin cell lines differing only in their metastatic potential, the team dialed down the effects of eIF2A. They found that in cancer cells, three-dimensional tumor spheres stopped growing and migration across a scratch in the culture dish slowed dramatically. Yet protein manufacturing was barely affected, overturning the idea that eIF2A launches protein synthesis. “Using a melanoma cell model consisting of nontumoral melanocytic Mel-ST cells and their metastatic counterpart obtained by H-Ras transformation, we unexpectedly find minimal effects of eIF2A depletion on translation,” they wrote.
To hunt for an alternative role, the researchers pulled eIF2A out of the cell using a molecular fishing line and catalogued which protein partners it was attached to. Many turned out to be components of the centrosome, a molecular structure that arranges microtubules and orients cells during movement. When eIF2A was absent, the centrosome often pointed the wrong way as cells tried to advance.
Further experiments revealed eIF2A works to preserve parts of the centrosome so it points the cell in the right direction during movement. The protein’s tail is critical for the cell’s migration power. Trimming the tail affected the cell’s ability to move and could be a potential drug-friendly target.
“The tail behaves like scaffolding cement, holding key parts of the melanoma’s cellular compass in place so that malignant cells can navigate their way out of the primary tumor,” said first author Jennifer Jungfleisch, PhD.
![Melanoma cells [Jennifer Jungfleisch/Centro de Regulación Genómica]](https://www.genengnews.com/wp-content/uploads/2025/08/low-res-20-300x300.jpeg)
“…our findings uncover a role of eIF2A in promoting centrosome composition and migration without overtly regulating translation,” the scientists concluded. “These findings challenge the traditional view of eIF2A as solely a translation initiation factor and open avenues for understanding the complex mechanisms contributing to melanoma metastasis.”
The authors further note that eIF2A dependence only emerges after malignant transformation, suggesting a therapeutic window that might spare healthy tissues. However, more work needs to be done to see how disrupting the protein’s behavior works in tissues and animal models. They acknowledged, “Further research is needed to clarify the molecular mechanisms by which eIF2A regulates centrosome composition and orientation. Studying the role of eIF2A in the migration of other cancer types could reveal broader implications for targeting eIF2A therapeutically.”
The post Melanoma Cells in Mice Steered by Protein’s Unexpected Function appeared first on GEN – Genetic Engineering and Biotechnology News.