Characterization of adsorbent material
Scanning Electron Microscopy (SEM) analysis of AC-ZnO-NH3 composite
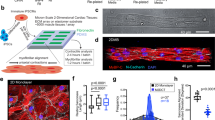
The SEM image of the AC-ZnO-NH3 composite was captured at a magnification of x400, as shown in Fig. 2. The SEM image of the AC-ZnO-NH₃ composite reveals a highly porous structure with interconnected cavities and a rough surface morphology. These features are critical for enhancing adsorption efficiency, as the porosity increases the accessible surface area and active binding sites for methylene blue (MB) molecules14. The presence of these pores suggests the material’s capability for enhanced mass transfer and increased contact efficiency, critical for processes like catalysis, filtration, or adsorption.
The surface morphology of the composite appears rough and uneven, indicating the presence of a variety of features, such as small particles and irregularly shaped aggregates. This roughness could contribute significantly to the overall surface area, further improving the material’s adsorption capabilities15. The irregularity in surface texture is likely a result of the synthesis process, which might promote enhanced active sites for chemical interactions.
Additionally, the uneven surface texture and non-uniform particle size distribution (ranging from 14 to 33 μm) further contribute to the composite’s functionality. Smaller particles (< 20 μm) provide a higher surface-to-volume ratio, facilitating rapid adsorption kinetics, while larger particles may enhance structural stability. However, heterogeneous particle sizes can lead to agglomeration, reducing active site availability16. Smaller particles generally provide a larger surface area relative to their volume, which facilitate greater interaction with target molecules17. Conversely, larger particles might affect material packing and flow properties, potentially influencing the material’s performance in practical applications. This observation aligns with Sachin et al., who demonstrated that uniform dispersion of ZnO nanoparticles within carbon matrices minimizes aggregation and optimizes adsorption-photocatalytic synergy18.
Recent studies, such as Ahmed et al. (2024) revealed that porous architectures in Magnetic oxide nano-porous adsorbents improve mass transfer and pollutant capture by maximizing contact between adsorbent surfaces and target contaminants1920.
Elemental composition and EDX analysis of the AC-ZnO-NH3 composite
The Energy Dispersive X-ray (EDX) analysis revealed the detailed elemental composition of the AC-ZnO-NH3 composite, highlighting the primary elements, their mass percentages, and atomic percentages. Figure 3 and Table 5 provide valuable insights into the structure and functionality of the composite. The composite is primarily composed of carbon, oxygen, iron, and zinc, with trace amounts of other elements such as sodium, magnesium, calcium, manganese, nickel, and copper. Each element contributes uniquely to the composite’s properties for example carbon constitutes 29.31% of the mass and 48.15% of the atomic composition. This high content reflects the presence of activated carbon (AC), which serves as the structural backbone of the composite. The activated carbon offers a large surface area and a porous structure, enhancing its adsorption efficiency for dye removal and water purification21. Oxygen represents 30.20% of the mass and 37.24% of the atomic composition. Its presence is attributed to oxygen-rich functional groups, including hydroxyl (-OH), carboxyl (-COOH), and carbonyl (C = O). These functional groups enhance the adsorption capacity by providing active binding sites. Additionally, oxygen is associated with zinc oxide (ZnO) component in the composite, further contributing to its functionality22. Zinc accounts for 5.40% of the mass and 1.63% of the atomic composition. The presence of zinc confirms the inclusion of ZnO, a semiconductor known for its excellent adsorption properties. Besides, elements such as sodium, magnesium, calcium, manganese, nickel, and copper were detected at concentrations below 2%. For example, calcium (Ca) was found at 1.15% mass and 0.57% atomic percentage, while copper (Cu) was present at 0.45% mass and 0.14% atomic percentage. These trace elements may arise from raw material impurities or synthesis conditions. The oxygen-containing functional groups improve the interaction between the composite surface and the target molecules, such as MB dyes, by providing specific binding sites23.
Fourier Transform Infrared (FTIR) analysis
The FTIR spectrum of the AC-ZnO-NH₃ nanocomposite (Fig. 4) reveals a variety of surface functional groups that play critical roles in adsorption and photocatalytic degradation of methylene blue (MB). The analysis was conducted in the spectral range of 4000–400 cm⁻¹, and the observed peaks are confirmed that the signals at 3856 cm⁻¹ and 3755 cm⁻¹ peaks correspond to the O–H stretching vibrations, indicating the presence of surface hydroxyl groups. Such groups enhance hydrogen bonding with dye molecules and improve surface polarity, facilitating stronger interactions during adsorption24. The peak at 3406 cm⁻¹ indicates to N–H stretching, this peak confirms the successful incorporation of amine groups through ammonia doping. These groups increase surface basicity and provide active sites for electrostatic interactions with the cationic MB molecules25. While, the peak at 3135 cm⁻¹ represents C–H stretching in aromatic compounds26suggesting the presence of π-conjugated carbon frameworks within the activated carbon. These aromatic structures participate in π–π stacking interactions with the aromatic rings of MB dye, enhancing surface contact and dye removal efficiency. A weak absorption at 2375 cm⁻¹ attributed to C = O stretching, typically associated with carboxylic acid groups. These functional groups contribute to hydrogen bonding with polar molecules and improve dye adsorption27. And the peak at 1611 cm⁻¹ assigned to C = C stretching vibrations of aromatic structures28further confirming the graphitic domains of the carbon structure and their role in π–π interactions. The peak at 1399 cm⁻¹ corresponds to C–N stretching, indicating the presence of secondary amines, which can also contribute to electrostatic attraction and dye complexation29. The peaks at 889 cm⁻¹ and 793 cm⁻¹ are attributed to C–H bending in aromatic and aliphatic compounds, supporting the complex hybrid surface chemistry of the composite. A distinct peak 427 cm⁻¹ corresponding to the Zn–O stretching vibration, confirming the successful integration of ZnO nanoparticles30. ZnO plays a crucial role in photocatalytic degradation by generating reactive oxygen species (ROS), including hydroxyl radicals (•OH) under sunlight irradiation31. The synergy between these functional groups enhances the overall performance of the AC-ZnO-NH₃ nanocomposite. While polar and ionizable groups (e.g., –OH, –NH₂, –COOH) facilitate adsorption via hydrogen bonding and electrostatic interactions, the presence of Zn–O sites support photocatalytic activity, making the material effective for both dye capture and degradation.
X-ray diffraction analysis
X-ray diffraction (XRD) is a widely used analytical method to investigate the crystal structure and phase composition of materials32. The X-ray diffraction (XRD) pattern of the synthesized AC-ZnO-NH₃ composite shows distinct peaks that reflect its partially crystalline nature (Fig. 5). The intense peaks at 2θ ≈ 25.28° and 26.43° correspond to the characteristic reflections of ZnO and possibly graphitic carbon structures33. Additional peaks observed at lower angles (5°–15°) may be attributed to interlayer spacing within the carbon matrix or the influence of ammonia functionalization. The peak broadening observed throughout the diffractogram suggests the formation of nanocrystalline domains. Crystallite sizes were calculated using the Scherrer equation, yielding values in the range of approximately 70–130 Å (7–13 nm), confirming the nanoscale structure of the ZnO particles. The presence of low microstrain values indicates minimal lattice distortion and confirms the structural stability of the material after modification34. These results support the successful synthesis of a hybrid AC-ZnO-NH₃ nanocomposite, in which ZnO nanoparticles are well-dispersed within the porous carbon matrix.
Crystallite size calculation using the Scherrer equation
The XRD pattern of the AC-ZnO-NH₃ composite revealed distinct peaks, indicating the presence of crystalline domains within the structure. Among the most intense reflections, the peaks at 2θ = 25.28° and 2θ = 26.43° were selected for crystallite size estimation using the Scherrer equation, which is defined as35:
$$D = frac{{K * lambda }}{{beta * cos theta }}$$
(8)
Where D is the crystallite size (in nm), K is the shape factor (typically 0.9), λ is the X-ray wavelength (Cu Kα = 1.5406 Å), β is the full width at half maximum (FWHM) in radians, and θ is the Bragg angle (half of 2θ, in degrees).
As an example, for the peak at 2θ = 26.43°, the following steps were applied: θ = 13.215°, Estimated FWHM = 0.3° → β = 0.3 × π/180 ≈ 0.00524 rad, cos(13.215°) = 0.9745.
– Substituting into the equation:
D = (0.9 × 1.5406)/(0.00524 × 0.9745) ≈ 271.7 Å = 27.17 nm.
However, based on the experimental software fitting results, the crystallite sizes of key peaks such as those at 25.28° and 26.43° were calculated as 72.95 Å (7.3 nm) and 130.36 Å (13.0 nm), respectively. These values are consistent with a nanocrystalline structure, confirming that ZnO is present in the form of nanoparticles embedded in the carbon matrix36.
The relatively broad nature of the peaks supports this conclusion and suggests a moderate level of lattice strain, as also indicated by the calculated microstrain values. This nanoscale architecture is advantageous for enhancing the surface area and active sites, which is crucial for both adsorption and photocatalytic applications.
RSM design for MB removal efficiency using AC-ZnO-NH3 composite
Interactive effects of process variables on MB removal efficiency using AC-ZnO-NH3 composite
Understanding the interactive effects of process variables is crucial for optimizing the removal efficiency of methylene blue (MB) dye using AC-ZnO-NH3 composites. This study explores the non-linear relationships and synergies between factors such as contact time, adsorbent dosage, pH, and initial dye concentration, providing insights into their combined influence on adsorption performance.
In Fig. 6a, the interaction between two factors contact time (A) and adsorbent dosage (B) on the removal efficiency of methylene blue (MB) dye using an AC-ZnO-NH3 composite. The response (R1) represents the percentage of MB dye removed from the aqueous solution. The x-axis represents contact time (A), and the y-axis represents adsorbent dosage (B). Both are coded variables, meaning their actual values are scaled for the RSM analysis, typically ranging from − 1 (low) to + 1 (high). The legend shows the range of actual factor levels. For example, all points on the ‘92’ line correspond to approximately 92% MB dye removal. These curved contours are a crucial element signifying the interaction effect between the variables.
The curved nature of the contour lines unequivocally demonstrates a significant interaction between contact time and adsorbent dosage. A simple additive model wouldn’t result in curved contours; instead, the curves showcase that the impact of one factor (dosage, for example) depends strongly on the level of the other factor (contact time).
The combined effect of moderate contact time and moderate dosage is more significant than their individual effects. The optimal removal zone lies in the heart of the plot, slightly leaning towards increased dosage (higher positive B value) with moderately increased time.
In Fig. 6b, the interaction effect of contact time (A) and pH (D) on the removal efficiency of methylene blue (MB) dye using an AC-ZnO-NH3 composite.
Contour plots showing the effect of adsorbent dose and time (a), pH and time (b), pH and adsorbent dose (c), pH and initial concentration of MB (d), initial concentration of MB and adsorbent dose (e).
The curved contour lines are strong indicators of a significant interaction effect between contact time (A) and pH (D). The response doesn’t simply increase or decrease linearly with changes in A or D; instead, it is dependent upon the relationship between both values. The region with the highest removal (dark red, above 98%) isn’t located at the extreme high values of A or D but, rather in the central region, suggesting that maximal MB dye removal requires an optimal combination of contact time and pH that fall towards increased coded time and pH values. Slight shifts to extremely high pH will be less effective. At high contact times with a relatively low pH value, the removal rate decreases notably, compared to the potential rate at moderate pH, illustrating diminishing returns as either condition shifts away from ideal levels.
The curved contour lines clearly indicate a strong interaction between contact time (A) and pH (D). The effect of changing one factor (contact time) on the MB dye removal efficiency isn’t consistent across all levels of the other factor (pH). The highest MB removal efficiency occurs in the region of positive (higher level of both A and D, indicating more time and a higher pH. However, there’s a clear diminishing return as approach the maximum value of A, while keeping D high, and if D were decreased to lower pH value for the same period of A there’s also a dramatic decrease in R1. The optimum is not on the boundaries, emphasizing the importance of the combined effect rather than extreme levels of either factor. The contour lines begin to flatten or compress towards the upper and lower ends, suggesting diminishing returns from extremely high or low values of contact time or pH. The non-linearity evident in the contour line curvature highlights the complexities of this interaction effect, requiring optimization efforts rather than relying on simplistic adjustments of the contact time or pH levels only. The change in each level must be coordinated for a truly optimized value of MB dye removal.
In Fig. 6c, the interaction effect of adsorbent dosage (B) and pH (D) on the removal efficiency (R1) of methylene blue (MB) dye. The curved nature of the contour lines indicates a significant interaction between adsorbent dosage and pH. This means that the impact of changing one variable depends strongly on the level of the other variable. The highest MB removal efficiency (near 100%) isn’t found at either extreme of the dosage (B) or pH (D) range, instead lying closer to increased B and D. This suggests an optimal region of the combination of moderate-to-high values, creating synergy in these variables rather than maximum dosage alone or high pH levels.
As move away from the central optimal region toward extreme high or low values of either factor (dose or pH), the removal efficiency decreases. For example, excessively high dosages past an optimum might lead to reduced dye removal; potentially, aggregation of the particles can occur or perhaps a threshold has been passed resulting in the saturation of reactive sites or causing issues that prevent efficiency from scaling. The curved lines clearly indicate that the relationship between the two factors and the MB dye removal isn’t linear, implying an intricate balance required between dose and pH for maximal effectiveness.
In Fig. 6d, The interaction effect between initial dye concentration (C) and pH (D). The curved contour lines strongly suggest a significant interaction effect between initial dye concentration and pH on the MB removal. The efficiency is not simply a linear sum of the effects of individual changes in dye concentration and pH, and the relationship must be considered as the change in each of the values of concentration and pH change in combination. Maximum MB removal (around 100%) is not at the extremes of either variable but rather within a specific zone characterized by: relatively higher pH values (positive values of coded D). Relatively lower initial dye concentrations (negative to slightly positive values of coded C). Going above 0 increases removal to approximately 95%, but above this point dramatically decreases removal as values of C increase, indicating this zone as close to the optimum. As move from this central optimum area to the extremes (higher dye concentrations or lower pH), removal efficiency considerably declines, indicating the significant diminishing returns to scaling a singular variable. The curved contours make clear this isn’t a straightforward relationship. A simple additive model would result in parallel lines, so the complex interactions between dose and pH levels strongly imply there’s a need for coordinated optimization rather than single variable changes.
In Fig. 6e, the interaction between the adsorbent dosage and initial dye concentration.
This means that the impact of altering one factor (dosage or concentration) isn’t constant but significantly depends on the level of the other factor. The highest removal efficiency (red region, near 100%) isn’t found at the extreme high levels of either factor.
The optimum is found more in areas with high values for both dosage and initial concentration; however, exceedingly high values are less efficient and result in substantially less dye removal (yellow/green region) once the sweet spot is surpassed. A modest decrease in either factor notably decreases efficacy as well, suggesting a fairly precise ‘sweet spot’ is required for maximum efficacy, which demands finely tuned values for the adsorbent dosage (B) and initial dye concentration (C). The contour lines curve, bunching together or becoming more densely packed as one moves away from the central zone of highest efficacy toward the outer limits of dosage or dye concentration. This highlights that adding extremely large dosages or starting concentrations does not yield proportionally greater removal; in fact, the increase may dramatically harm the overall outcome, illustrating the importance of working within an area around a carefully modeled and studied optimized dosage and concentration zone. The curved lines strongly suggest a complex, non-linear relationship between the two factors and dye removal efficiency. A simplistic additive model (where the individual effects of concentration and dose are simply added) would result in straight parallel lines. However, the significant curves observed clearly demonstrate this non-linear model, which must be properly optimized using more controlled experimentation to avoid confounding issues when modeling and making projections of final outcomes. This visualization demonstrates that maximizing methylene blue (MB) removal isn’t merely about using the maximum dosage and/or concentration; an optimized result would require finding and operating very near this very precisely tuned optimal point where dosage and initial dye concentration synergize efficiently for high removal. Operating in this sweet spot may allow the highest outcome without added or wasted adsorbent materials or exceeding dye removal ability from concentration saturation that leads to poor MB dye removal once saturation or confounding issues come into effect. Beyond this zone, performance could diminish rapidly10.
Perturbation plot of MB dye removal efficiency (R1)
Figure 7 presented the perturbation plot, this perturbation plot, a component of Response Surface Methodology (RSM) analysis, visualizes the impact of small changes (perturbations) in each process variable on the MB dye removal efficiency (R1). The plot shows how the response (R1) varies when each factor is individually changed, keeping the other factors constant at their reference point (the central point indicated by the black dot). The x-axis represents the deviation from this reference point in coded units (+ 1 is the highest value tested, −1 is the lowest). The y-axis is the percentage of MB dye removed.
The impact of A (Contact Time) perturbation increasing contact time (moving right along the A line) slightly decreases the MB dye removal. This is likely due to effects reaching an equilibrium point in MB adsorption, after which adding extra contact time wouldn’t produce much additional benefit37. This curve may demonstrate a subtle slope toward further improvement rather than pure negative slope only.
While, B (Adsorbent Dosage) near flat slope of line B implies that modest alterations around the reference point don’t significantly alter the MB removal percentage. This doesn’t imply there’s no relationship overall, simply that in the zone tested around the center there is no demonstrable increased MB removal from slight dose changes. This could imply the need for larger scale changes around this region for a greater benefit to move to substantially higher dosages may offer larger positive benefits38.
Whereas, C (Initial Dye Concentration) an increase in initial dye concentration (moving to the right along line C) shows a small increase in MB removal. At least in the values tested immediately surrounding this region a slight increase may enhance removal. More dramatically, larger values will have much more demonstrably impacted results; there may even be thresholds passed around this area where the addition of concentration no longer yields any improved dye removal at the level tested around this region.
In addition, D (pH) similar to contact time, modest changes in pH values, around the reference point (positive values shown moving right along line D), show a slight decrease in the MB dye removal efficiency. It may show an optimal point lying just beyond what the reference values tested include.
Overall, the perturbation plot indicates that, among small deviations around the reference conditions tested in this set of initial trials, only the initial dye concentration shows some influence. Modest increases near the middle reference values slightly improve dye removal efficiency; however, much larger increases would be required before a change in this response to greater benefits is observed. On the other hand, increasing the contact time and pH slightly decreases efficiency around the current conditions tested.
A comparison between the actual and predicted values of MB dye removal efficiency (R1)
Figure 8 shows a comparison between the actual and predicted values of MB dye removal efficiency (R1) obtained from the RSM model. The diagonal line indicates perfect agreement between predicted and actual values. The color of each point indicates the level of R1 for that particular run. The primary way to assess model accuracy is to observe how closely the points cluster around the diagonal line. The majority of the points lie relatively near the diagonal, indicating that the RSM model predicts the experimental results reasonably well within the specific conditions tested. Specifically, most results tested closely resemble the predictions. At low R1 values, the point at the far lower-left represents a considerable underprediction. This signifies potential systematic biases for lower removal rates within this experimental region that were not predicted from the modeling itself, suggesting the need for reevaluation or a correction model for conditions with substantially lower than expected removal rates.
At high R1 values, the points toward the upper right of the diagram have varied results showing generally better fit for the conditions that are closer to 100% in removal efficiency and relatively poor fit near this area for points less than this maximal point, indicating improved reliability in the higher tested efficiency zone of the composite.
The color gradient also assists with identification of trends or potential biases among the various data point results. So, the greatest underprediction may potentially result more from results that are at low MB removal rates, and the improved predictions observed mostly relate to improved predictions close to high efficacy results. This could imply issues of precision may be related specifically to those runs at or approaching those values.
Normal probability plot of residuals assesses
The overall appearance of the plot implies near conformance and adequate normalcy despite notable and demonstrably evident outliers near higher and lower extremes. To improve, the modeling should first attempt to resolve systematically why these conditions do not produce aligned results by closely scrutinizing testing conditions at the higher and lower efficacy values to discover why there may be larger and unexpectedly distributed error amongst this subset of runs39. Doing so would yield more refined and reliable results, allowing for predictions within the specific areas tested using improved modeling in those outlier conditions. It would also potentially increase the accuracy, dependability, and predictability needed to precisely extrapolate results from the overall modeled outcome, considering diverse reaction parameters, thus enabling more accurately modeled predictions of likely MB dye removal (Fig. 9).
Box-Cox plot
The Box-Cox in Figure 10 helps determine the optimal power transformation for the response variable (R1, MB dye removal efficiency) to achieve normality and improve the RSM model’s performance40. The plot displays the log-likelihood (Ln (Residual SS)), which is a measure of model fit, as a function of the Box-Cox transformation parameter (lambda, λ). The λ-axis shows different power transformations. λ = 1 indicates no transformation (linear scale), while values other than 1 correspond to power transformations. For instance, λ = 0.5 represents taking the square root, λ = 0 represents a log transformation, and λ=−1 implies taking the reciprocal. The log-likelihood (Ln (Residual SS)) axis indicates the quality of the model fit. Lower values are preferred as they represent a smaller residual sum of squares, meaning the model fits the data more accurately after applying the transformation. The plot identifies the best-fitting power transform by visualizing a confidence interval around the minimum of this function. The graph shows the minimum likelihood around λ = 1, with the vertical blue line indicating a 95% confidence interval that completely encompasses λ = 1, meaning no transformation is needed.
Based on this analysis, applying no transformation to this response (R1) may likely yield the best results; given there is already near conformance in other parameters this test appears to strengthen this interpretation further, thus this Box-Cox plot result lends weight to the hypothesis this current model assumptions may be perfectly suitable and already well aligned in terms of generating acceptable levels of fit and efficacy for MB dye removal rate without the need for this type of transformation itself.
Assessment of model performance and limitations in ANOVA
From the ANOVA analysis the overall model has an F-value of 0.7215 and a p-value of 0.7142, indicating that the model is not significant. The closest to significance is the interaction term BC (p-value = 0.0819), but it is still above the threshold.
The coefficient estimates indicate the relative effect of each factor: C (initial dye concentration) has the largest positive effect (coefficient = 2.98), although its confidence interval includes zero, indicating statistical insignificance. Interaction term BC (initial dye concentration and dosage) has a relatively high coefficient (9.66), but again, it is not statistically significant.
All variance inflation factor (VIF) values are below 10, indicating no severe multicollinearity among factors. The negative Predicted R2 (−1.9723) suggests that the model performs worse than using the overall mean of the response. This indicates very poor predictive ability. The low Adjusted R2 (−0.1869) further implies overfitting or inadequate representation of the data. With a value of 3.5644, the model does not meet the recommended threshold (Adeq Precision > 4), indicating an insufficient signal-to-noise ratio for reliable predictions or optimization. As determined earlier, none of the terms in the model are statistically significant. This weakens the model’s utility for identifying critical factors or designing a robust process.
Adsorption study by batch process
Influence of pH
The adsorption of MB dye on the AC-ZnO-NH3 nanocomposite is highly pH-dependent. Figure 11 showed that at pH 3, the removal efficiency was 75.27%. The acidic environment likely caused protonation of functional groups on the adsorbent surface, such as hydroxyl (-OH) and carboxyl (-COOH) groups41. This protonation reduces the availability of negatively charged sites on the adsorbent surface, limiting electrostatic interactions between the adsorbent and the cationic MB dye molecules42. Hence, the removal efficiency at this pH is moderate. While, at pH 5, the removal efficiency improved to 83.21%, indicating more favorable adsorption conditions. As the pH increases, the extent of protonation of the adsorbent’s surface decreases, enhancing the availability of negatively charged functional groups43. These groups interact more effectively with the positively charged MB dye, increasing the adsorption efficiency. The removal efficiency at pH 7 dropped slightly to 75.09%. Atneutral pH, the surface charge of the adsorbent may begin to neutralize further due to a balance between positively and negatively charged functional groups44. This neutralization could decrease the electrostatic interactions necessary for efficient MB dye adsorption. Additionally, competition between hydroxide ions (OH⁻) and the dye molecules for adsorption sites may occur. At pH 9, the removal efficiency reached its maximum at 94.40%, demonstrating optimal conditions for adsorption. In this alkaline environment, the adsorbent surface is predominantly negatively charged, enhancing electrostatic attraction to the cationic MB dye45. Moreover, the higher pH may reduce dye aggregation in the solution, increasing the availability of individual dye molecules for adsorption. This condition maximizes the interaction between the adsorbent and MB dye.
Also, Oxygen-containing functional groups on the activated carbon and zinc oxide nanoparticles contribute significantly to adsorption. These groups interact chemically and physically with the dye molecules, enhancing removal efficiency46. MB dye is stable in both acidic and alkaline conditions, but higher pH may prevent dye aggregation, leading to more efficient adsorption.
So, the optimal removal efficiency (94.40%) was observed at pH 9, likely due to enhanced electrostatic interactions and reduced competition for adsorption sites. These findings emphasize the importance of pH optimization in designing efficient dye removal systems and highlight the effectiveness of the AC-ZnO-NH3 composite in environmental remediation applications.
Effect of initial concentration
The results demonstrate how varying the initial MB dye concentration affects the percentage removal efficiency (% removal) and adsorption capacity (qe, mg/g) of the AC-ZnO-NH3 composite as presented in Fig. 12. At low initial concentration (10–30 ppm), the removal efficiency (% removal) is high across this range, peaking at 99.13% at 30 ppm. Adsorption capacity increases with concentration, reaching 123.92 mg/g at 30 ppm. This trend suggests that at lower concentrations, the available active sites on the adsorbent are sufficient to adsorb nearly all MB dye molecules. The slight variation in % removal indicates that the adsorption process remains highly effective due to the ample surface area and functional groups provided by the AC-ZnO-NH3 composite12. At medium initial concentration (40–50 ppm), at 40 ppm, % removal decreases slightly to 96.25%, with a corresponding decrease in qe to 120.31 mg/g while, at 50 ppm, % removal drops further to 85.70%, and qe decreases to 107.13 mg/g. This decline in efficiency is likely due to the saturation of adsorption sites as the dye concentration increases47. The reduced availability of active sites relative to the number of dye molecules results in lower removal efficiency. While, at high initial concentration (100 ppm), the removal efficiency recovers slightly to 98.48%, and qe stabilizes at 123.10 mg/g. This recovery indicates that despite the high concentration of dye, the composite maintains excellent adsorption performance. The direct sunlight may contribute to photodegradation of MB, which complements the adsorption process at higher concentrations48.
At low concentrations, the number of dye molecules is significantly lower than the available active sites on the composite49. This results in high % removal and efficient adsorption. As concentration increases, competition among dye molecules for the limited active sites intensifies, leading to a gradual decline in % removal and qe. The presence of ZnO nanoparticles in the composite, combined with direct sunlight, may contribute to photocatalytic degradation of MB dye50. This mechanism becomes more evident at higher concentrations, explaining the improved removal efficiency at 100 ppm. The maximum adsorption capacity (qe=123.92 mg/g) observed at 30 ppm underscores the effectiveness of the AC-ZnO-NH3 composite for MB dye removal. This high capacity is attributed to the synergy between activated carbon (providing high surface area) and ZnO introducing photocatalytic activity and additional binding sites50.
Effect of adsorbent dosage
The study highlights that adsorbent dosage significantly affects both % removal and adsorption capacity (qe). Figure 13 showed that the results reveal how increasing the adsorbent dosage influences the percentage removal efficiency and adsorption capacity (qe, mg/g). At low adsorbent dosage (10 mg) the percentage removal is 93.65%, with the highest adsorption capacity (qe=117.06 mg/g, qe = 117.06 mg/g). At this low dosage, the number of available adsorption sites is limited but sufficient to achieve high dye removal efficiency for the given dye concentration51. This also leads to a high qe, as each gram of adsorbent is responsible for adsorbing more dye molecules. While, at medium adsorbent dosage (20–30 mg), increasing the dosage to 20 mg improves % removal to 98.20%, but qe drops significantly to 61.37 mg/g. At 30 mg, % removal remains high at 97.47%, nonetheless qe declines further to 40.61 mg/g. This decrease in qe with increasing dosage is attributed to the aggregation of adsorbent particles, which reduces the effective surface area and available active sites52. Additionally, some adsorption sites remain underutilized as the dosage increases, leading to a decline in adsorption capacity.
While, at high adsorbent dosage (40–50 mg), at 40 mg, % removal peaks at 99.21%, indicating that an optimal number of adsorbent particles are present to maximize dye removal. However, qe continues to decrease, reaching 31.00 mg/g. At 50 mg, % removal remains high at 98.34%, but qe drops further to 24.58 mg/g. The diminishing returns in qe suggest that increasing the adsorbent dosage beyond a certain point led to site saturation53. While additional particles are present, their adsorption potential is not fully utilized due to the limited number of dye molecules in the solution. A dosage of 40 mg achieves the highest % removal (99.21%) as optimal performance. While increasing the dosage improves % removal, it reduces qe, indicating diminished efficiency of the adsorbent per unit mass at higher dosages54. At low adsorbent dosages, the limited number of particles maximizes the utilization of active sites, resulting in high qe. However, as the dosage increases, the surface area becomes excessive relative to the dye concentration, leading to underutilized active sites55. Higher dosages can lead to particle clustering or aggregation, reducing the effective surface area and access to adsorption sites56. This effect can contribute to the reduction in qe. Once the dye molecules saturate the available active sites, increasing the adsorbent dosage has minimal impact on % removal. This is evident at dosages of 40–50 mg, where the % removal remains relatively constant. The presence of ZnO in the composite, combined with direct sunlight, may enhance dye degradation, complementing the adsorption process. This effect may contribute to the high % removal observed at all dosages.
Effect of contact time
The adsorption efficiency of MB dye on the AC-ZnO-NH3 composite is highly time-dependent, with rapid initial adsorption followed by a slower approach to equilibrium.
Figure 14 showed that the results highlight the influence of contact time on the adsorption efficiency of MB dye removal, as measured by the percentage removal (% removal). During initial phase (10–20 min), the first 20 min, the % removal increased from 84.62 to 85.92%, indicating a rapid adsorption process. In this phase, the availability of a large number of active sites on the adsorbent’s surface facilitates the fast uptake of MB dye molecules from the solution. The slight plateau observed between 10 and 20 min suggests that the dye molecules are beginning to saturate the readily accessible sites. At intermediate phase (30 min), at 30 min, the % removal decreases to 79.28%, showing an anomalous dip in efficiency. This decline could result from dye molecule desorption due to local equilibrium fluctuations or temporary saturation of active sites, possibly caused by the lack of agitation and uneven distribution of dye molecules in the solution57.While, steady state phase (60–120 min), from 60 to 120 min, the % removal improves significantly, reaching 91.99% at 60 min and 93.29% at 120 min. This steady increase reflects the gradual diffusion of MB molecules to less accessible adsorption sites within the adsorbent’s porous structure58. Photocatalytic activity under direct sunlight, facilitated by ZnO nanoparticles, may also enhance dye degradation during this period, contributing to the increased removal efficiency. While, at equilibrium phase (180 min), at 180 min, the % removal reaches its peak at 97.40%. By this point, most active sites on the adsorbent are occupied, and the adsorption rate has significantly slowed due to reduced availability of free dye molecules in the solution59.
Maximum % removal (97.40%) is achieved at 180 min, indicating that equilibrium is reached at this time. The intermediate decline in removal efficiency at 30 min highlights the need for agitation or optimized conditions to maintain consistent adsorption rates. Over time, as these sites become saturated, the adsorption rate slows, and the process transitions to a diffusion-controlled phase where dye molecules penetrate deeper into the adsorbent’s pores. ZnO nanoparticles in the composite enhance the removal process through photocatalytic degradation of MB dye under direct sunlight60. This mechanism complements the physical adsorption by breaking down dye molecules into smaller, less complex species. The lack of agitation in the setup could contribute to localized concentration gradients, slowing the overall adsorption rate and causing uneven distribution of dye molecules. This effect may explain the dip in removal efficiency at 30 min. As equilibrium is approached, the remaining dye molecules must travel longer distances to reach unoccupied sites deep within the adsorbent structure, leading to a gradual slowing of the adsorption process61.
Effect of different light source and photocatalytic activity
The efficiency of the photocatalytic degradation process is not only dependent on the material itself but also influenced by several external factors such as the nature and intensity of the light source, the solution’s pH level, and the existence of additional substances or co-contaminants in the medium. Investigating the role of these parameters is essential for gaining a deeper and more accurate understanding of the overall performance of the photocatalytic system.
In this study, the degradation potential of green-synthesized ZnO nanoparticles toward methylene blue was evaluated under different lighting conditions (as shown in Fig. 15) at initial dye concentration (50 ppm), contact time (180 min), adsorbent dosage (20 mg). In a typical experiment, a known amount of ZnO nanoparticles was added to a 50 mL solution of MB dye at a specific concentration and pH. The mixture was not agitated for a predetermined contact time The findings reveal that both the irradiation source and the nanoparticle concentration have a substantial impact on the photocatalytic outcome.
Among the tested light sources, natural sunlight led to the highest removal efficiency, reaching 92.7%. This high activity can be attributed to the ultraviolet (UV) portion of sunlight, which plays a major role in activating the ZnO nanoparticles. The broad wavelength range of sunlight, which spans both the UV and visible regions, enhances the generation of reactive oxygen species (ROS), thereby boosting the breakdown of methylene blue molecules more effectively than other light sources76. The ultraviolet portion of the light spectrum plays a key role in initiating the photocatalytic process by promoting electrons from the valence band to the conduction band of ZnO nanoparticles. This transition results in the generation of electron-hole pairs, which subsequently engage in a series of redox reactions at the catalyst surface. These reactions lead to the formation of highly reactive species, particularly hydroxyl radicals and other reactive oxygen species (ROS), which are primarily responsible for attacking and decomposing methylene blue molecules77.
The observed degradation of methylene blue under different lighting conditions confirms the photocatalytic nature of the process. In the absence of light, the reaction showed an almost negligible degradation rate (0.72%), emphasizing that light energy is essential to initiate and sustain the photocatalytic activity of ZnO nanoparticles. When exposed to UV light alone, the degradation reached a moderate level (17.8%), indicating the fundamental role of ultraviolet radiation in activating the photocatalyst. However, the comparatively higher efficiency observed under sunlight suggests that other spectral components, such as visible light, may enhance the photocatalytic performance synergistically with UV radiation.
Room light led to a limited degradation rate (7.7%), which implies that either the light intensity or the spectral composition typical of indoor lighting lacks the necessary energy to efficiently excite the ZnO photocatalyst. Although visible light contributes less significantly compared to UV, it still plays a supportive role in the degradation process. Through photon absorption, visible light can promote electrons to higher energy states within the conduction band of ZnO, potentially enhancing the generation of reactive oxygen species (ROS), albeit at a lower rate.
ZnO is recognized as a semiconductor with notable photocatalytic capabilities. When illuminated by photons with energies exceeding its bandgap, it generates electron-hole pairs. These charge carriers are key participants in surface redox reactions, interacting with water and oxygen molecules adsorbed on the catalyst surface to produce ROS such as hydroxyl radicals, which are the main drivers of oxidative decomposition of dye molecules like methylene blue.
The superior degradation efficiency under sunlight is attributed to its wide spectral range, encompassing both UV and visible regions. This broad excitation enables a higher population of conduction band electrons and valence band holes, thereby increasing the formation of ROS and enhancing the breakdown of organic contaminants78. The limited degradation observed under dark and ambient light conditions reinforces the conclusion that the process is predominantly photocatalytic. In the absence of adequate light energy, the excitation of electrons from the valence to the conduction band in ZnO nanoparticles is greatly reduced, which in turn restricts the formation of reactive oxygen species (ROS) necessary for dye breakdown. Consequently, the degradation remains minimal under such conditions.
Extending the contact time between methylene blue and the ZnO nanoparticles generally enhances degradation efficiency, as it allows more opportunities for interaction between dye molecules and reactive species. Nonetheless, the rate of degradation does not remain uniform throughout the process. Initially, degradation proceeds rapidly due to the abundance of accessible dye molecules, but it may decelerate over time as these molecules are depleted and the remaining ones become more difficult to degrade, either due to limited diffusion or more stable chemical structures.
Furthermore, it is important to consider that both the ZnO nanoparticle concentration and the dye concentration have optimal thresholds. Beyond these levels, photocatalytic efficiency may decline. Excessive nanoparticle loading can lead to light scattering and particle agglomeration, which reduce the active surface area and hinder light penetration. Similarly, high dye concentrations may result in intense competition for active sites and incident photons, ultimately decreasing the overall degradation efficiency13. so, the factors influencing the rate of degradation over time include the initial dye concentration, light intensity, and the efficiency of mass transfer within the system.
Sorption isotherms
The adsorption isotherms provide critical insight into the equilibrium relationship between methylene blue (MB) dye molecules and the AC-ZnO-NH₃ composite under sunlight exposure. Three isotherm models Langmuir, Freundlich, and Temkin were evaluated using nonlinear regression fitting, with results presented in Fig. 16 and summarized in Tables 6 and 7 in addition 2–4 S. The experiments investigated the effect of initial MB dye concentrations ranging from 10 to 100 ppm on adsorption efficiency. These models were assessed based on their respective regression coefficients (R²), predicted adsorption values (qₑ), and error functions including SSE, APE%, χ², and EABS, which reflect model reliability and predictive power.
Langmuir isotherm model
The Langmuir isotherm assumes monolayer adsorption on a homogenous surface, where adsorption sites have uniform energy62. The Langmuir model assumes monolayer adsorption on a homogeneous surface with identical adsorption sites and energies63. As shown in Fig. 16a, the model exhibits an excellent fit to the experimental data, with a correlation coefficient R² = 0.999, indicating a very strong linear relationship between predicted and observed values. The theoretical maximum adsorption capacity ((:{Q}_{m})) is 106.38 mg/g aligns well with experimental values, confirming that the AC-ZnO-NH3 surface is saturated at higher concentrations. This reflects the upper limit of dye molecules that can be adsorbed onto the AC-ZnO-NH3 composite surface. Langmuir Constant (:{K}_{a}=9.40times:{10}^{-3}) is related to the affinity of the binding sites for the dye molecules64. A higher value indicates stronger binding between the dye and the adsorbent. While, the accuracy of (:{Q}_{e})-predicted compared to (:{Q}_{e})-experimental and the low error functions suggest the Langmuir model fits well (Table 5).
Adsorption Isotherm for MB dye onto AC-ZnO-NH₃ Composites (a) Langmuir, (b) Freundlich, (c) Temkin Isotherm model.
The model’s reliability is confirmed by low SSE values, such as 11.87, and very small absolute percentage errors (APE%), ranging from 0.02 to 0.31% across the tested concentrations (Table 5). Additionally, χ² values remain low (e.g., 4.62 at 10 ppm, 7.22 at 100 ppm), suggesting minimal deviation between predicted and experimental qₑ values. This model best represents the adsorption process, particularly at medium and high MB concentrations.
Freundlich isotherm model
The Freundlich model assumes multilayer adsorption on a heterogeneous surface65. Despite this, the model demonstrated a poor overall fit, with R² = 0.875 and high deviations at both low and high concentrations. For instance, at 10 ppm, the predicted qₑ was 23.04 mg/g versus an experimental value of 121.84 mg/g, resulting in SSE = 23.21 and χ² = 80.12. Similar discrepancies are seen at higher concentrations, such as SSE = 17.63 and χ² = 90.38 at 100 ppm (Table 6; Fig. 16b).
The Freundlich model accounts for multilayer adsorption on a heterogeneous surface. The model equation includes two key constants: KF, representing adsorption capacity, and 1/n, which indicates adsorption intensity. The value of (:1/n=0.524) indicates favorable adsorption ((:0<1/n<1)), but the adsorption intensity is moderate, reflecting the composite’s strong surface interactions but limited capacity for multilayer adsorption66. At low concentrations (10 ppm), the Freundlich model captures the heterogeneity of the surface, as reflected in reasonable APE and (:{chi:}^{2}) values. The (:1/n=0.524) parameter indicates favorable adsorption, consistent with a moderately strong interaction between the dye molecules and the adsorbent. At higher concentrations (50–100 ppm), the Freundlich model fails to predict the observed adsorption capacity accurately, with substantial deviations ((:{chi:}^{2}=90.38) at 100 ppm) (Table 6). This highlights the model’s limitation in systems where monolayer adsorption becomes dominant, as multilayer adsorption effects diminish. The high error metrics of APE% > 0.80% and EABS exceeding 100 in some cases—indicate that the Freundlich model cannot accurately describe adsorption at saturation or high surface coverage, where monolayer adsorption becomes dominant.
Temkin isotherm model
The Temkin model considers the linear decrease in adsorption energy with coverage, accounting for indirect adsorbate–adsorbent interactions. As shown in Fig. 16c, the Temkin model provides a weak fit to the experimental data, with a very low R² = 0.169, suggesting it is not well-suited for modeling MB adsorption in this system63. Nevertheless, it provides qualitative insight into the role of energetic interactions. The calculated Temkin parameters include B = 7.76, and A = 220013.63 L/g at 10 ppm. However, large errors were observed, particularly at high concentrations: for example, at 100 ppm, the predicted qₑ was 93.31 mg/g versus an experimental value of 123.10 mg/g, with SSE = 6.57, APE% = 6.57, and an exceptionally high χ² = 531.63 (Table 7).
These results indicate that, although the Temkin model accounts for changes in adsorption energy, it fails to accurately predict equilibrium uptake, especially when adsorption is controlled by monolayer or saturation effects.
Also, Table 6 presented the absolute percentage error (APE%) values range from 0.02 to 0.31%. These low values indicate that the Langmuir model effectively predicts the adsorption capacity across different concentrations67. Also, the chi-Square ((:{chi:}^{2})) values demonstrate the closeness of the predicted and experimental data. Lower (:{chi:}^{2}) indicates a better fit of the model68. Error absolute bias sum (EABS) values quantify the deviation between predicted and experimental data. Smaller values, such as 2.31 at 50 ppm, highlight the reliability of the Langmuir model in describing the adsorption process69.
Kinetic models
Understanding the adsorption kinetics is essential for elucidating the mechanisms that govern the interaction between MB dye and the AC-ZnO-NH3 composite. In this study, the Lagergren First-Order, Pseudo-Second-Order kinetic and intraparticle diffusion models were applied to assess the rate-limiting steps and to predict adsorption behavior. The results show significant differences in how well these models describe the experimental data, revealing critical insights into the adsorption mechanism.
The Lagergren first-order model is typically applied to systems where physical adsorption (physisorption) is dominant. It assumes that the adsorption rate is proportional to the number of unoccupied active sites on the adsorbent’s surface70. The results in Table 7; Fig. 17 (A&B) show the low correlation coefficient ((:{R}^{2}=0.419)) and underestimated (:{q}_{e}) (calc.) value of 0.60 mg/g indicate that this model poorly describes the adsorption process. The small (:{k}_{1}) value of 17.96 × 10(:{}^{3}) min(:{}^{-1}) suggests that the adsorption rate is slow and that physisorption alone cannot account for the rapid dye removal observed experimentally. The composite exhibits chemisorption behavior (strong chemical interactions), which the first-order model does not account for. The heterogeneous nature of the adsorbent surface due to ZnO integration introduces complexities, such as multiple adsorption mechanisms, which deviate from the assumptions of this model.
The pseudo-first-order model of MB dye on AC-ZnO-NH3 under adsorption (A) and photocatalytic condition (B).
Under photocatalytic conditions, qe equal 1.01 mg/g and k1 = 0.01036 min⁻¹, in addition, that R2 = 0.594 moderate but still unsatisfactory. Consequently, the pseudo-first-order model does not adequately describe the MB removal process, suggesting that the mechanism is not solely dependent on dye concentration but involves more complex surface interactions.
The pseudo-second-order model assumes that chemisorption is the rate-limiting step, where adsorption depends on electron exchange or covalent bonding between the adsorbate and the adsorbent71. The pseudo-second-order model achieves a near-perfect fit with (:{R}^{2}=0.998), (Fig. 18A; Table 7), demonstrating its ability to accurately describe the adsorption kinetics of MB dye on AC-ZnO-NH3. The calculated (:{q}_{e}) of 2.46 mg/g is much closer to the experimental value compared to the first-order model, highlighting the dominance of chemisorption72. High-rate constant ((:{k}_{2})) value of 96.52 × 10(:{}^{3}) g/mg·min indicates rapid adsorption, consistent with the synergistic effects of activated carbon and ZnO nanoparticles73. In photocatalytic conditions, the AC-ZnO-NH₃ composite exhibited a calculated equilibrium adsorption capacity (qe) of 2.45 mg/g, with a pseudo-second-order rate constant (k₂) of 0.03423 g/mg. min. The model fit was exceptionally strong, as indicated by a high correlation coefficient (R² = 0.996), confirming the reliability of the pseudo-second-order model in describing the kinetics of the process (Fig. 18B).
The pseudo-second-order model of MB dye on AC-ZnO-NH3 under adsorption (A) and photocatalytic condition (B).
Based on the results presented in Table 7 and Fig. 19A&B, the intra-particle diffusion model was applied to evaluate the adsorption behavior of methylene blue (MB) dye onto AC-ZnO-NH₃ under both adsorption and photocatalytic conditions. This model characterizes the time-dependent nature of intra-particle diffusion and indicates that the adsorption process is governed by diffusion when the rate is influenced by the movement of the adsorbate and adsorbent toward one another74.
The intraparticle diffusion model of MB dye on AC-ZnO-NH3 under adsorption (A) and photocatalytic condition (B).
Under adsorption conditions, the diffusion rate constant was found to be Kdif = 0.05, indicating a relatively slow transport of dye molecules into the pores. Under photocatalytic conditions, this value increased to Kdif = 0.18, suggesting that the diffusion process was significantly enhanced by photocatalytic activation, which may facilitate the movement of dye molecules toward active sites75. While, the parameter C reflects the boundary layer thickness. A higher value of C = 0.25 under adsorption implies a greater resistance due to the boundary layer, indicating that external mass transfer might be influencing the overall process. In contrast, a much lower value of C = 0.03 under photocatalytic conditions suggests a minimal boundary layer effect, which can be attributed to the enhanced surface reactions and mass transport promoted by photocatalysis.
In addition, under adsorption conditions, the R² value was relatively low (R2 = 0.64), indicating that the intra-particle diffusion model did not fully account for the kinetic behavior and that other mechanisms might be contributing. However, under photocatalytic conditions, an excellent correlation was observed (R2 = 0.997), indicating that the model closely describes the adsorption process, with intra-particle diffusion likely being the dominant rate-controlling step76.
Finally, the results indicate that the pseudo-second-order kinetic model best describes the adsorption behavior under both conditions. This is supported by the high correlation coefficients (R2 = 0.998 for adsorption and R2 = 0.996 for photocatalysis), suggesting that the process is chemisorption-driven and depends on the availability of active sites on the surface of the material. In contrast, the pseudo-first-order model showed poor fitting, with relatively low R2 values (0.419 and 0.594), indicating that it does not adequately represent the mechanism controlling the adsorption process. The intra-particle diffusion model provided further insight into the mass transfer behavior within the pores. Under adsorption-only conditions, the relatively low R2 = 0.64 suggests that intra-particle diffusion is not the sole rate-limiting step. However, under photocatalytic conditions, the model exhibited excellent agreement with the data (R2 = 0.997), indicating that intra-particle diffusion becomes more dominant, likely due to enhanced surface activation and increased reactivity facilitated by light irradiation. Additionally, the calculated qe values from the pseudo-second-order model showed excellent agreement with the experimental data, reinforcing the model’s accuracy in predicting adsorption capacity.
Suggested mechanism of MB dye adsorption onto AC-ZnO-NH3 composites
Adsorption Mechanism of MB with AC-ZnO-NH₃ Composite (Expert-Level).
The adsorption mechanism of methylene blue (MB) onto the AC-ZnO-NH₃ composite is governed by a synergistic interplay of multiple interactions, involving both physical and chemical processes. Based on the comprehensive characterization (FTIR, SEM, Zeta potential), kinetic modeling, and supported by recent studies34,77,78,79the following mechanism is proposed (Fig. 20):
Schematic illustration of the methylene blue removal process by the AC-ZnO-NH₃ composite, showing the adsorption mechanisms.
- 1.
Electrostatic Interaction:
- 2.
At alkaline pH (optimum at pH 9), the surface of the AC-ZnO-NH₃ composite acquires a negative charge due to the deprotonation of surface functional groups (–OH, –COOH, –NH₂), which facilitates strong electrostatic attraction toward the cationic MB molecules.
- 3.
π–π Electron Donor–Acceptor Interactions:
- 4.
The aromatic π-electron systems of both the MB dye and the graphitic domains of activated carbon engage in π–π stacking. This interaction is particularly stabilized due to the planar nature of MB and the conjugated carbon structure, promoting adsorption via van der Waals forces and molecular orbital overlap.
- 5.
Hydrogen Bonding:
- 6.
Functional groups such as –OH and –NH₂ introduced through ammonia doping enhance hydrogen bonding with the nitrogen and sulfur atoms of MB, increasing the interaction strength and stability of the adsorbed complexes.
- 7.
Photocatalytic Degradation (ROS Generation):
- 8.
Upon exposure to sunlight, ZnO nanoparticles within the composite generate reactive oxygen species (ROS), including hydroxyl radicals (•OH) and superoxide anions (O₂•⁻), which attack the aromatic rings of MB. This leads to the fragmentation of dye molecules into smaller, less toxic or mineralized byproducts (e.g., CO₂, H₂O), as demonstrated in prior ROS studies. This photocatalytic degradation not only contributes to MB breakdown but also regenerates active sites on the adsorbent surface.
- 9.
Surface Complexation and Chemisorption:
- 10.
The pseudo-second-order kinetic model (R² = 0.998) suggests that chemisorption, involving electron transfer or surface complex formation between MB and the active functional groups on the adsorbent, is a dominant mechanism. Nitrogen functionalities introduced via ammonia serve as electron-rich sites that facilitate MB coordination.
In summary, MB removal by AC-ZnO-NH₃ involves a hybrid mechanism combining physisorption (π–π stacking, hydrogen bonding, and electrostatic attraction) with chemisorption and photocatalytic oxidative degradation. This dual functionality not only enhances removal efficiency but also supports dye mineralization under environmentally friendly conditions.
Suggestion A cost Estimation approach of methylene blue dye removal via AC-ZnO-NH3 composite
Accurately estimating the cost of a study is essential for effective budgeting and resource allocation. The cost of research projects can vary significantly based on a variety of factors, including the materials and reagents used, experimental setup, personnel, and data analysis requirements80. In this context, a detailed breakdown of potential costs for a study assessing the efficiency of an Activated Carbon-Zinc Oxide-Ammonia (AC-ZnO-NH3) composite for removing methylene blue dye from wastewater is provided (Table 8).
The cost was determined using a mix of various sources, such as commercial websites for equipment and chemicals, previous research reports, and references from academic or research institutions that use these materials and equipment in similar experiments. The breakdown considers different categories of expenses that may arise and the factors influencing them. The cost of materials and reagents depends significantly on the type of supplier, quantity, and required specifications. The cost of 1 kg of commercially available high-quality activated carbon ranges from $100 to $300. Prices vary depending on the source and purity of the material. The cost of 1 kg of high-purity zinc oxide nanoparticles ranges from $150 to $500. The cost is affected by the particle type, size, and purity. Also, the cost of 1 L of concentrated ammonium hydroxide (28–30%) ranges from $15 to $30. The cost may vary depending on the quantity required. The cost of 100 g of high-purity methylene blue dye ranges from $50 to $150, influenced by the required quantity and purity. This category includes acids, bases, and solvents like hydrochloric acid, sodium hydroxide, and ethanol. The estimated cost for these reagents is between $100 and $200, depending on the required quantity.
The estimated total cost for materials ranges from $415 to $1180 for the lower estimate and $1015 to $2130 for the higher estimate, depending on experimental requirements. The cost of equipment is influenced by the availability of existing infrastructure in the laboratory or the need to purchase new equipment. The cost of new glassware may range from $200 to $500, but this can be reduced if existing equipment is available.
If the balance is already available in the lab, costs can be minimized. If not, the cost ranges from $300 to $2000, depending on the specifications as presented in Table 1. If spectrophotometer available at the institution, costs can be minimized. Otherwise, purchasing a new spectrophotometer could cost several thousand dollars. If the pH meter is already available, the cost is minimal. If not, a new pH meter may cost between $50 and $300. If magnetic stirrer/hot plate available, costs can be minimized. Otherwise, a basic purchase might cost between $50 and $200. If the drying oven is available, the cost is low. If a new one is required, the cost could range between $300 and $1000. Using SEM/EDX and FTIR centralized facilities for material analysis can significantly reduce costs. External analysis services could range from $500 to $2000. If existing equipment (total for experimental setup) is used, the total cost could range from $750 to $1400. This estimate may increase if new equipment or external analysis is required.
Labor and personnel costs depend on the required work hours and the number of researchers involved (principal investigator, research assistants). For a study involving a single research assistant, labor costs might range from $3000 to $10,000. However, if more assistants or significant effort is needed, costs could range from $10,000 to $50,000. Labor costs could range from $3000 to $50,000, depending on project requirements.
Miscellaneous expenses and additional costs could range from $100 to $300 based on experimental needs. This category could range from $1100 to $3300, depending on publication and experimental needs. The costs could range from $2715 to $6400 for a smaller-scale project requiring fewer resources and effort. The costs could range from $5820 to $25,730 for a larger project requiring more specialized equipment, labor, and external analysis.
These estimates are based on various assumptions about quantities and specifications. To obtain a more accurate estimate, it is important to review specific supplier prices and laboratory conditions. Additionally, a comprehensive project proposal and consultation with the relevant lab personnel will help refine the budget and provide a more precise cost estimate.
Evaluation of reusability and regeneration for dye removal
The regeneration experiments conducted to assess the reusability of the Multifunctional AC-ZnO-NH3 Composite demonstrated significant initial adsorption efficiency but revealed a decline in performance over successive regeneration cycles. Table 9 showed the dye removal efficiencies recorded were 88.571% for first stage, 37.778% for second stage and the third stage was 27.937%. The initial high removal efficiency highlights the composite’s excellent adsorption capability during the first cycle. However, the sharp decrease in removal efficiency in subsequent cycles suggests the presence of irreversible adsorption sites, incomplete desorption, or structural/chemical degradation of the composite material during regeneration processes81. Correspondingly, the sharp decline in removal efficiency after the first stage could be attributed to during the initial adsorption, strong chemisorption interactions may have occurred between the dye molecules and the active sites of the composite material, leading to incomplete regeneration. Residual dye molecules might block active adsorption sites, reducing available surface area and adsorption efficiency in subsequent cycles82. Repeated exposure to dyes and regeneration chemicals might alter the surface chemistry of the composite, reducing its affinity for the dye molecules83.
Comparison of adsorption capacity
Comparing the adsorption efficiency with other adsorbent materials in previous investigations revealed that AC-ZnO-NH₃ nano composites in present study exhibited good adsorption capacity due to the synergistic effect of the nono zinc and activated carbon, and displayed higher adsorption capacity than robust adsorbent combinations such as AC –cellulose and CS-g-GEL/SWCNTs (Table 10). Although some composite materials such as CH–Mt/PANI showed higher adsorption capacity; but AC-ZnO-NH₃ nano composites remain cost-efficient material.