Scientists at the University of Chicago Pritzker School of Molecular Engineering (UChicago PME) have engineered polymer-based nanoparticles that form with a simple temperature shift. The team reports that the new nanoparticles, described in a study “Thermoreversibly Assembled Polymersomes for Highly Efficient Loading, Processing, and Delivery of Protein and siRNA Biologics” in Nature Biomedical Engineering, self-assemble at room-temperature in water and, because of these gentle conditions, can deliver proteins, which are unstable in many existing nanoparticle formulations.
“What excites me about this platform is its simplicity and versatility. By simply warming a sample from fridge temperature to room temperature, we can reliably make nanoparticles that are ready to deliver a wide variety of biological drugs,” said co-senior author Stuart Rowan, PhD, the Barry L. MacLean Professor for Molecular Engineering Innovation and Enterprise, University of Chicago Pritzker Molecular Engineering, and a staff scientist Argonne National Laboratory.
Protect delicate drugs
Nanoparticles are key to protecting delicate drugs like RNA and proteins from being degraded in the body before they reach the right cells. Lipid nanoparticles (LNPs), made of fatty molecules, enabled the COVID-19 mRNA vaccines, for instance. But LNPs rely on alcohol-based solvents and sensitive manufacturing steps—making them poorly suited for protein delivery and hard to scale, notes the research team.
“We wanted to make a delivery system that could work for both RNA and protein therapies because right now most platforms are specialized for just one,” said first author Samir Hossainy, a University of Chicago Pritzker School of Molecular Engineering (PME) graduate student. “We also wanted to make it scalable, without needing toxic solvents or complicated microfluidics.”
Hossainy hypothesized that polymer-based nanoparticles could offer a more robust, customizable alternative. He outlined the required characteristics; the immune system will only respond to particles with certain sizes, shapes, and charges. Then, he used chemical tools to begin designing new nanoparticles from scratch.
After trying, and fine-tuning, more than a dozen different materials, he found one that worked. In cold water, the polymer—and any desired protein—remained dissolved. But when heated to room temperature, the polymer self-assembled into uniformly sized nanoparticles (polymersomes) surrounding the protein molecules.
“Our particle size and morphology are dictated only by the chemistry of the polymers that I designed from the bottom up,” explained Hossainy. “We don’t have to worry about different particle sizes forming, which is a challenge with a lot of today’s nanoparticles.”
Freeze-dried and stored without refrigeration
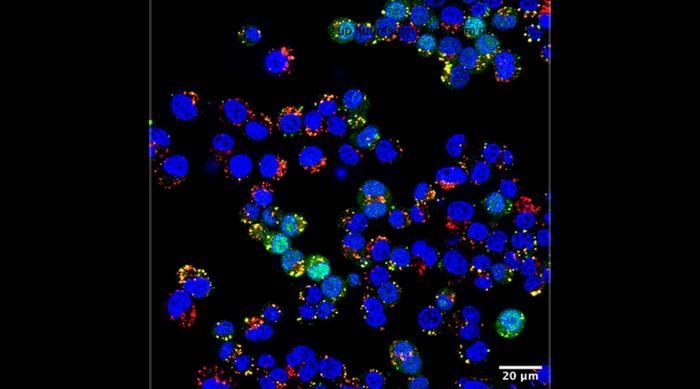
To test the new polymersomes, Hossainy worked with colleagues in Rowan’s lab as well as with former University of Chicago PME Jeffrey Hubbell, PhD, now at New York University. First, they showed that the particles can encapsulate more than 75% of protein and nearly 100% of short interfering RNA (siRNA) cargo—far higher than most current systems. These polymersomes can also be freeze-dried and stored without refrigeration until needed.
In the context of vaccination, Hossainy and his collaborators found that the polymersomes could effectively carry a protein and, when injected into mice, lead the animals’ immune systems to generate long-lasting antibodies against that protein. Another experiment showed that the nanoparticles could also carry proteins designed to prevent an immune response in the context of allergic asthma. And a third showed that injecting polymersomes into tumors could block cancer-related genes and suppress tumor growth in mice.
“The exciting thing is that we didn’t need to tailor a different system for each use case,” said Hossainy. “This one formulation worked for everything we tried—proteins, RNA, immune activation, immune suppression, and direct tumor targeting.”
One of the biggest advantages of the new polymersomes over current LNPs is the potential for low-tech, decentralized production, continues Hossainy, adding that he imagines being able to ship freeze-dried formulations of the nanoparticles to anywhere in the world. When they need to be used, they can be mixed in cold water, warmed up, and will be ready to deliver to patients.
“Being able to store these dry drastically improves the stability of the RNA or protein,” he points out.
The group is continuing to work on fine-turning the particles to carry more types of cargo, including messenger RNA like that used in the COVID-19 vaccines (generally much larger than the siRNA used in the current trial). They also plan to collaborate on preclinical trials to apply the polymersomes to real-world vaccine or drug delivery challenges.
The post Self-Assembling Nanoparticles at Room Temp Could Transform Drug Delivery appeared first on GEN – Genetic Engineering and Biotechnology News.