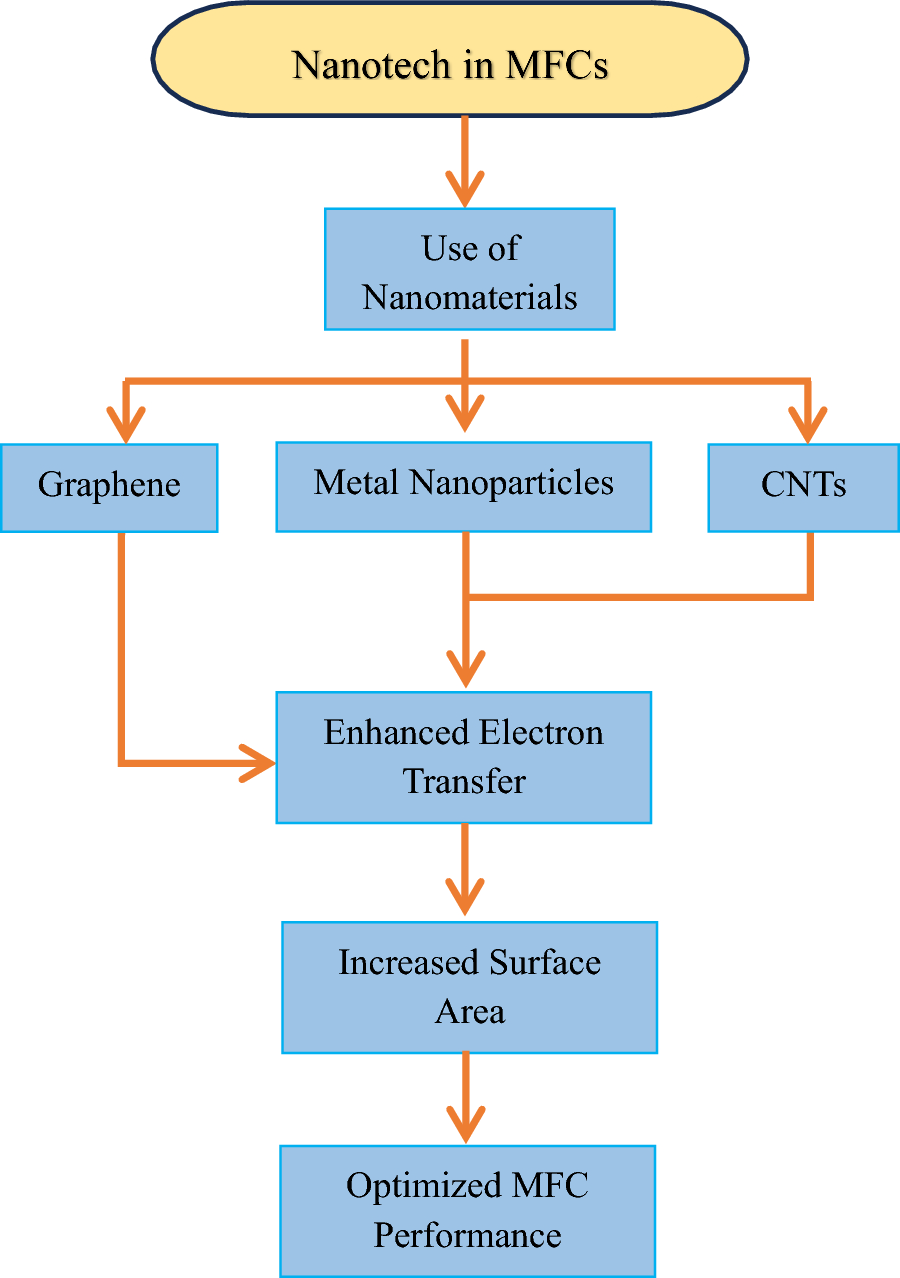
- Review
- Open access
- Published:
Biotechnology for Biofuels and Bioproducts volume 18, Article number: 74 (2025) Cite this article
Abstract
Food loss and waste (FLW) generated by unsustainable linear food systems are major contributors to greenhouse gas (GHG) emissions. Although microalgal lipid production has advanced significantly for applications such as biofuels and high-value polyunsaturated fatty acids (PUFAs), the use of FLW as an alternative feedstock to cultivate lipid-rich microalgal biomass within a circular bioeconomy remains insufficiently explored. This review critically evaluates the feasibility of converting FLW into nutrient-rich media for microalgae cultivation, with particular focus on its effects on biomass productivity and lipid accumulation. Pre-treatment methods for food waste are essential to enhance nutrient recovery, especially of carbon sources, and significantly influence subsequent microalgae cultivation. These methods affect the bioavailability of key nutrients, particularly the carbon-to-nitrogen-to-phosphorus (C/N/P) ratio, which regulates metabolic pathways involved in lipid biosynthesis. Despite encouraging laboratory-scale outcomes, large-scale implementation remains constrained by feedstock heterogeneity, high energy demands during harvesting and lipid extraction, and regulatory challenges. To overcome these barriers and facilitate scale-up, this review highlights integrative strategies such as metabolic engineering, automated cultivation systems, and a closed-loop microalgae-based biorefinery. Moreover, life cycle assessment (LCA) is emphasized as a tool to assess environmental performance and inform policy decisions, supporting alignment with Sustainable Development Goals (SDG 12 and SDG 13).
Graphical Abstract

Background
Food loss and waste (FLW) occur throughout the food supply chain, including production, packaging, transportation, and storage processes. Globally, approximately 1.3 billion tonnes of food are lost or wasted annually, representing one-third of all food produced for human consumption [1]. For instance, in 2019 alone, an estimated 931 million tonnes of food were wasted by households, retailers, restaurants, and other food services [2]. These large quantities of waste not only exacerbate food insecurity, affecting around 2.4 billion people [3], but also contribute significantly to climate change, as FLW accounts for 8–10% of global greenhouse gas (GHG) emissions each year [2]. Moreover, over 90% of food waste is still disposed of together with municipal solid waste through landfilling and incineration [4]. These conventional food waste management (FWM) methods further intensify environmental impacts by releasing large amounts of GHGs and other pollutants. Landfilling promotes anaerobic decomposition of organic matter, resulting in the emission of methane (CH4), a potent GHG with a global warming potential 28–34 times greater than that of carbon dioxide (CO2) over a 100-year time frame [5]. Although incineration reduces the physical volume of waste, it releases large amounts of CO2 and other harmful emissions such as nitrogen oxides (NOx), particulate matter, and volatile organic compounds (VOCs), which pose risks to both environmental and public health [6]. In addition, incomplete combustion produces highly toxic compounds such as dioxins and furans, which further exacerbate environmental pollution [7]. Therefore, urgent action is needed to develop integrated and sustainable solutions that simultaneously address food waste, mitigate environmental impacts, and resolve associated social and economic challenges.
Food wastes are suitable alternative culture media for microalgae cultivation because they contain high concentrations of organic carbon, nitrogen, phosphorus, and other essential micronutrients [8]. Organic carbon serves as both an energy and carbon source, particularly under mixotrophic or heterotrophic cultivation. In contrast, phototrophic systems rely on light to drive photosynthetic carbon fixation. Nitrogen and phosphorus are important for the synthesis of biomolecules (e.g., proteins, nucleic acids, and lipids), which are crucial for maintaining cell structure and function. Furthermore, micronutrients (e.g., trace elements and vitamins) in food waste support enzymatic activity and regulate metabolism, thereby promoting optimal microalgae growth and biomass productivity [9]. Based on these advantages, converting nutrient-rich food waste into a usable culture medium not only reduces the burden of waste disposal, but also facilitates nutrient recovery for microalgal biomass production [10]. However, food waste typically requires pre-treatment before it can be used for microalgae cultivation, which may incur additional costs. Therefore, pre-treatment costs should be considered when assessing the economic feasibility of this approach. Moreover, a comprehensive evaluation of the economic feasibility must account for both upstream processes (i.e., microalgae cultivation) and downstream processes (i.e., harvesting, dewatering, extraction, and purification of valuable compounds) of the resulting microalgal biomass [11], all of which play a critical role in determining the overall cost-effectiveness and commercial potential of the system.
Maximizing the value of microalgal biomass through efficient extraction and conversion into high-value products is essential, as it improves the overall cost-efficiency and environmental performance of the microalgae-based biorefinery system. Among the various components that can be derived from microalgal biomass, lipids have attracted significant interest due to their high commercial potential across industries such as biofuels, pharmaceuticals, nutraceuticals, and cosmetics [12]. Microalgae-derived lipids present opportunities for cost advantages when integrated into a cascading biorefinery strategy, in which lipids are first extracted and the residual biomass is further utilized to recover proteins and pigments [13]. To ensure the viability of this approach, mild or non-destructive lipid extraction methods are required to preserve the spent algal biomass for subsequent recovery of heat-labile compounds (e.g., carotenoids, chlorophyll, and phycobiliproteins). Suitable techniques include supercritical CO2 extraction, pressurized fluid extraction, and natural deep eutectic solvents (NADES) or enzymatic-assisted extraction at moderate temperatures [14, 15]. The recovery of these preserved heat-labile compounds significantly enhances the economic feasibility of microalgae-derived lipid production. For instance, microalgae-derived carotenoids can command prices exceeding $7,500/kg, whereas synthetic carotenoids are typically priced between $3,000 and $4,000/kg [16]. In addition to economic considerations from recovering high-value co-products, researchers can enhance the bio-productivity of microalgae-derived lipid production through environmental manipulation such as light intensity, CO2 concentration, temperature, nutrient availability, salinity, and heavy metal stress. Furthermore, advanced metabolic and genetic engineering techniques can be employed to promote targeted lipid accumulation under controlled conditions [17].
Within the circular bioeconomy framework, microalgae-based valorization of food waste offers a promising strategy for resource loop closure, generating high-value functional lipids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [18]. This strategy not only contributes to effective food waste management but also integrates carbon sequestration with the production of nutrient-rich compounds. Importantly, while several United Nations Sustainable Development Goals (SDGs) can be conceptually linked to this strategy, the most directly relevant are SDG 12 (Responsible Consumption and Production) and SDG 13 (Climate Action). Specifically, repurposing food waste into valuable microalgal lipids and bioproducts aligns with SDG 12 by promoting sustainable waste utilization, minimizing resource loss and advancing circular biorefinery. Concurrently, carbon fixation through microalgal photosynthesis and the reduction of CH4 emissions from decomposing waste directly contribute to SDG 13 by mitigating GHG emissions and supporting climate action efforts [19]. Although ancillary benefits like reducing pressure on marine fisheries or enhancing nutrition security exist, these are secondary outcomes rather than core objectives of this valorization pathway.
This review is based on a comprehensive literature search conducted primarily using Web of Science, Scopus, and Google Scholar databases for publications from 2015 to early 2025. Keywords included combinations of “food waste,” “microalgae cultivation,” “lipid production,” “biorefinery,” and “circular bioeconomy.” Priority was given to original research and recent reviews with clear relevance to food waste valorization pathways for microalgal lipid synthesis. Based on this body of literature, this review identifies a significant gap in current research, as most studies narrowly address individual aspects such as food waste pre-treatment, microalgal cultivation in waste-based media, or microalgal lipid biosynthesis in wastewater, without integrating them into a comprehensive closed-loop framework [8, 10, 20]. To bridge this gap, this review proposes a closed-loop, microalgae-based biorefinery framework that connects sequential stages of food waste valorization, including tailored waste pre-treatment, optimized microalgae cultivation using nutrient-rich hydrolysates, targeted lipid extraction, and cascading utilization of residual algal biomass. Moreover, this review further highlights the role of life cycle assessment (LCA), techno-economic analysis (TEA), AI-assisted process optimization, and supportive policy frameworks to address scalability barriers and facilitate the commercial implementation of microalgae-based biorefinery. By integrating these elements into a system-level closed-loop framework, this work provides essential insights for enabling a sustainable transition toward a circular bioeconomy in alignment with SDG 12 and SDG 13.
Food waste as a sustainable culture medium for microalgae cultivation
Utilizing food waste as alternative culture media for cultivating microalgae shows dual benefits of waste reduction and resource recovery. Organic nutrients (e.g., carbon, nitrogen, and phosphorus) derived from food waste can be supplemented as essential elements for microalgae growth [21], replacing synthetic media while achieving comparable or higher biomass concentration [22]. Unlike conventional cultivation strategies, food waste-based media introduce variable and complex matrices that require specific process interventions to enhance nutrient availability and maintain consistent microalgae growth. The heterogeneous composition of food waste necessitates pre-treatment to break down complex organics (e.g., carbohydrates, proteins, lipids) into bioavailable forms (e.g., sugars, amino acids) and disrupt intrinsic structure of solid food waste composition. Such pre-treatments not only facilitate nutrient accessibility, but also influence downstream lipid productivity and biorefinery compatibility. Therefore, choosing an appropriate pre-treatment method is essential for balancing efficiency, cost, and sustainability in the cultivation process using food waste.
Overview of pre-treatments and their effectiveness on nutrient recovery from food waste
Pre-treatment is the process of breaking down or modifying food waste to improve nutrient recovery, including bio-accessibility and digestibility for microalgae growth [23]. Accordingly, pre-treatment strategies should focus on enhancing the availability of key nutrients, particularly carbon sources such as monosaccharides, disaccharides, and volatile fatty acids, which affect subsequent biomass production and lipid biosynthesis in microalgae. Techniques such as enzymatic hydrolysis and mild thermal treatment are effective in releasing soluble sugars and organic acids from complex food waste, thereby facilitating carbon uptake by microalgae and directing metabolic flux toward lipid accumulation [24]. Figure 1 illustrates the major sources of food waste, including households, food processing industries, agriculture, restaurants and food services, as well as retail stores and supermarkets. It also presents the characteristics of food waste and the corresponding common pre-treatment methods, which include physical (e.g., sorting, shredding, homogenization), chemical (e.g., acid/alkaline treatment), biological (e.g., enzymatic pre-treatments), and physicochemical (e.g., hydrolysis, sterilization) processes to enable effective bioconversion.
In general, physical pre-treatments for food waste include mechanical, ultrasonication, and microwave-assisted techniques [25]. Among these, ultrasonic pre-treatment of food waste involves applying high-intensity ultrasound waves (typically 20 kHz) to disrupt cell walls and membranes in organic compounds [26], thereby releasing intracellular components including nutrients and enzymes. For example, Liu et al. (2018) reported that ultrasonic treatment significantly increased soluble chemical oxygen demand (SCOD) and accelerated the reduction of volatile solids (VS), improving bio-accessibility and degradability of organic compounds in food waste [27]. Although sCOD and VS are not directly utilized as nutrients for microalgae cultivation, sCOD reflects the soluble carbon fraction available for microbial metabolism, and VS indicates the total organic matter content in food waste [28]. Furthermore, Joshi and Gogate observed that ultrasonication-induced cavitation increased the sCOD of food waste by 61.5%, indicating improved solubilization efficiency for anaerobic digestion [29].
Beyond increasing sCOD, ultrasonication has been shown to enhance the release of proteins, sugars, and other soluble nutrients. It can also disrupt lipid-containing structures, facilitating the release of intracellular lipids from lipid-rich waste [30]. However, the presence of excessive free oil in culture media may form surface films that block light and hinder gas exchange, thereby inhibiting microalgal growth [31]. Therefore, the objective of pre-treatment is not only to increase nutrient solubility, but also to convert complex compounds, including lipids, into bioavailable forms such as short-chain fatty acids (SCFAs) or glycerol that can be readily assimilated by microalgae [32].
In addition, microwave-assisted pre-treatment employs irradiation to enhance anaerobic co-digestion efficiency by facilitating the breakdown of complex organic compounds in food waste [33]. Although this process is primarily used to boost biogas production, it can indirectly benefit microalgae cultivation by increasing soluble carbon availability. According to the results of a study carried out by Yue and colleagues, the content of SCOD increased by approximately 41.46% (from 14,280 ± 590 mg/L to 20,200 ± 360 mg/L) after microwave pre-treatment [34]. Compared to conventional electric heating, microwave heating offers more rapid and uniform thermal energy delivery, improving the pyrolysis rate and overall solubilization efficiency [35]. Because of these advantages, microwave heating has been proposed as a potentially sustainable and efficient pre-treatment technology [36].
Chemical pre-treatment methods, including acid and alkaline treatments, modify food waste composition by breaking down complex structures (e.g., lignocellulose) into simpler sugars and polymers, thereby enhancing enzyme accessibility. However, these processes may concurrently generate inhibitory compounds, such as furans from acid hydrolysis or phenolic derivatives produced under alkaline conditions. To address this trade-off, careful optimization of pre-treatment parameters (e.g., pH, temperature, duration) is critical for balancing structural degradation with the reduction of inhibitor formation, ensuring efficient downstream bioconversion [37]. For instance, a study underscored that acid pre-treatment can increase the solubilization of carbohydrates, proteins, and lipids by 87%, 41%, and 135%, respectively. However, acid hydrolysis may release several inhibitors (e.g., acetic acid, furfural, hydroxymethylfurfural (HMF)) that negatively affect microbial kinetics and microalgal cultivation [36]. These improvements in process efficiency directly enhance the economic feasibility of producing biofuels and other bioproducts through microalgal conversion of nutrients derived from pre-treated food waste.
To be specific, acid pre-treatment employed acidic solvents such as inorganic acids (e.g., sulfuric acid (H2SO4), hydrochloric acid (HCl)) or organic acids (e.g., citric acid (C6H8O7), acetic acid (CH3COOH)) to treat food waste [36]. For example, a study on kitchen waste pre-treatment in Malaysia utilized dilute HCl and H2SO4 to break down larger polysaccharide molecules into smaller and fermentable sugars such as glucose and maltose from food waste, achieving an initial conversion efficiency of 42.4% [38], To overcome the limitations of inhibitor formation and improve sugar yields, sequential acid-enzymatic methods have been developed, combining hydrothermal, dilute acid, and enzymatic hydrolysis to significantly increase sugar recovery compared to acid pre-treatment alone. On the other hand, alkaline pre-treatment used alkaline solutions such as sodium hydroxide (NaOH) or aqueous ammonia (NH3·H2O) to treat food waste. Research conducted on anaerobic digestion of food waste found that using 1% calcium oxide (CaO) as an alkaline pre-treatment significantly enhanced the solubilization of organic matter, including fats, leading to enhanced biogas yields [39].
Biological pre-treatment methods, particularly enzymatic hydrolysis, are widely used in laboratory-scale FWM. Unlike traditional approaches that rely on costly purified enzymes (e.g., cellulases, proteases), enzymatic pre-treatment can derive advantage from crude and unprocessed enzymes obtained directly through biomass hydrolysis. These impure enzyme mixtures contain diverse enzymes and biomolecules, enabling broader substrate specificity compared to purified enzymes, which are often limited by their high specificity [36]. For instance, impure enzymes efficiently hydrolyze complex food waste components like proteins, carbohydrates, and lipids through synergistic interactions between different enzyme types and biomolecules present in the mixture, facilitating rapid degradation and conversion into valuable products such as volatile fatty acids (VFAs) [40]. The process typically involves extracting enzymes (e.g., cellulases, proteases, lipases, and amylases) from biomass sources through hydrolysis, collecting them without further purification, and directly applying them to food waste for rapid breakdown. In addition to enzymatic techniques, microbial fermentation improves biodegradability by utilizing bacteria or fungi to decompose complex organics. For example, Yousuf et al. (2018) investigated the impact of enzymatic pre-treatment on dark microbial fermentation of food waste, showing an increase in total solid content by 5% compared to aeration pre-treatment, highlighting its efficacy in waste valorization [41].
Thermal pre-treatment, especially hydrothermal processing, is widely acknowledged as an effective physio-chemical method for treating food waste. Based on a study conducted by Ding et al. (2017), the key findings indicated that treating food waste at 140°C for 20 min maximizes the solubilization of organic components, especially carbohydrates and proteins [42]. In summary, food waste pre-treatment strategies should prioritize the solubilization of fermentable sugars and amino acids that microalgae can metabolize efficiently, while minimizing the formation of inhibitory compounds and preserving the integrity of key nutrients.
Microalgae-driven mechanisms for GHG emissions mitigation: carbon assimilation and nutrient recycling pathways
Conventional FWM methods, such as landfilling and incineration, lead to large amounts of GHG emissions. Landfills produce CH4 due to anaerobic decomposition, while incineration releases large amounts of CO2 into the atmosphere, contributing directly climate change [4]. In contrast, microalgae-based valorization offers a biologically integrated solution by enabling carbon and nutrient recycling. Under mixotrophic cultivation, microalgae can simultaneously assimilate organic carbon from food waste hydrolysates and fix inorganic CO2 industrial emissions. This dual carbon uptake not only enhances biomass productivity, but also contributes to carbon sequestration. CO2 can be supplied through direct injection or flue gas sparging into photobioreactors, allowing integration with emission-intensive industries such as fermentation, bioethanol production, or fossil fuel combustion [43]. In addition, microalgae absorb nitrogen (N) and phosphorus (P) from food waste, reducing the release of pollutants including nitrous oxide (N2O) [44]. Therefore, it is essential to understand the mechanisms of carbon assimilation and metabolic pathways involved in nutrient recycling (i.e., nitrogen and phosphorus) in microalgae. Elucidating how microalgae regulate key metabolic pathways, such as the tricarboxylic acid (TCA) cycle, glycolysis, and nitrogen assimilation, in response to combined organic and inorganic carbon sources will support the development of optimized, environmentally sustainable FWM strategies.
Carbon assimilation from food waste by microalgae
Food waste contains various rich carbon sources including carbohydrates (e.g., glucose, fructose), proteins, and lipids (e.g., glycerol, fatty acids). During pre-treatment, these complex organic molecules are broken down into simpler compounds, such as monosaccharides, organic acids, and volatile fatty acids (VFAs). Microalgae utilize these compounds through heterotrophic (i.e., organic carbon) and autotrophic (i.e., inorganic carbon) pathways, enabling flexible metabolic responses to different carbon inputs [45]. Monosaccharides like glucose and fructose, which are abundant in fruit, vegetable, and bakery waste, are absorbed by microalgae through facilitated diffusion or active transport [46]. Glucose undergoes glycolysis, producing two adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NADH) per glucose molecule. Fructose enters glycolysis after conversion to fructose-1-phosphate by fructokinase and cleavage into dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G3P). These triose phosphates then enter the glycolytic pathway, generating equivalent energy molecules (2 ATP and 2 NADH per fructose molecule) [47].
Pyruvate, the end product of glycolysis, is converted to acetyl-coenzyme A (acetyl-CoA) in the mitochondria of eukaryotic microalgae, linking glycolysis to the TCA cycle. The TCA cycle produces additional NADH, flavin adenine dinucleotide (FADH₂), and guanosine triphosphate (GTP), which are used in oxidative phosphorylation to drive ATP synthesis, contributing to microalgal biomass growth and lipid biosynthesis [43]. Moreover, organic acids such as acetic acid and lactic acid, commonly derived from fermented food waste or dairy waste, enter the TCA cycle as intermediates. Ethanol and glycerol from beverage and processed food waste are converted into acetyl-CoA and G3P, respectively, and also enter central metabolism [48, 49].
In addition to heterotrophic carbon assimilation, microalgae fix inorganic carbon (CO2) through the Calvin cycle, a light-independent photosynthetic pathway in chloroplasts. The enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) catalyzes CO2 fixation with ribulose-1,5-bisphosphate (RuBP) to form two molecules of 3-phosphoglycerate acid (3-PGA). Subsequent reactions convert 3-PGA into G3P using ATP and NADPH from light reactions [50, 51]. Most of the G3P molecules are used to regenerate RuBisCO and maintain the cycle, and the remaining G3P is employed to synthesize organic compounds such as glucose, which are essential for the growth of microalgae. Figure 2 illustrates general metabolic pathways in microalgae involved in (a) carbon fixation via the Calvin cycle and (b) nutrient assimilation and recycling (nitrogen and phosphorus), which are activated following nutrient uptake.
Microalgal metabolic pathways for carbon fixation and nutrient assimilation activated by nutrient uptake nutrient uptake. Adapted from [56] with permission from Elsevier
Bicarbonate (HCO3−) from dissolved CO2 is another inorganic carbon source. Carbonic anhydrase catalyzes its conversion to CO2, which is then used for carbon fixation in the Calvin cycle [52]. Since the decomposition of organic compounds in food waste releases CO2, this CO2 can dissolve in the surrounding water and form HCO3−. Microalgae can utilize this carbon pool for photosynthesis [53]. Improving carbon assimilation efficiency is crucial for optimizing microalgae-based food waste valorization [54]. The type of food waste and its composition influence carbon availability, it is essential to select a suitable pre-treatment method to ensure effective carbon assimilation. Recent studies have used genetic engineering to enhance Calvin cycle efficiency for improving CO2 fixation in microalgae. For example, a study by Yang and teammates introduced a plastid transit peptide-fused enhanced green fluorescent protein (EGFP) into the chloroplast of Chlorella vulgaris. Subsequently, they transferred cyanobacterial fructose 1,6-bisphosphate aldolase (FBAld) into the Chlorella vulgaris using genetic manipulation techniques [55]. This innovative approach increased photosynthetic efficiency by more than 1.2 times by promoting RuBP regeneration and optimizing energy transfer within the chloroplast. As a result, these findings underscore the potential of genetic modifications to enhance carbon assimilation efficiency in microalgae, which could further improve their ability to utilize carbon sources from food waste.
Metabolism of nitrogen and phosphorus from food waste in microalgae
Food waste pre-treatment methods (e.g., hydrolysis, fermentation) play a critical role in enhancing the bioavailability of nitrogen (N) and phosphorus (P) for microalgae cultivation. Hydrolysis breaks down proteins into amino acids and ammonium, while fermentation releases organic acids that solubilize phosphorus compounds [43]. These pre-treated food waste form nutrient-rich media, enhancing nutrient uptake and microalgal biomass production. Organic nitrogen sources (e.g., amino acids) are degraded into inorganic nitrogen such as nitrate (NO3−) and ammonium (NH4+). Microalgae take up these inorganic compounds through specific membrane transporters and complete nitrogen assimilation within the cell to synthesize essential organic molecules like amino acids. Nitrogen metabolism in microalgae primarily involves in absorption and transformation of NO3− and NH4+ [57]. Among these, NO3− is catalyzed by nitric reductase to be reduced to nitrite (NO2−) and further converted to NH4+. Microalgae then absorb NH4+ through specific transporters located on the cell membrane. Once inside the microalgae cell, the assimilated nitrogen synthesizes amino acids primarily through the glutamine synthase/glutamate synthase (GS/GOGAT) pathway and is further involved in protein synthesis [58]. In addition, light intensity significantly affects nitrogen assimilation in microalgae by modulating the activity of enzymes involved in nitrogen uptake and reduction [59]. For instance, a study found that Chlorella pyrenoidosa achieved its highest growth rate achieved its highest growth rate (1.54 ± 0.29 d−1) and nitrogen removal efficiency under 7000 Lux, highlighting the regulatory role of light in nitrogen metabolism [60].
Phosphorus in food waste often exists in organic forms (e.g., phytic acid, nucleic acids). Food waste pre-treatment increases the solubility of phosphorus, primarily in the form of inorganic phosphate (Pi) ions, such as phosphate (PO43−), monohydrogen phosphate (HPO42−), and dihydrogen phosphate (H2PO4−) [61]. Figure 2(b) demonstrates the metabolic pathways of nitrogen and phosphorus in microalgae. For the metabolic pathway of phosphorus in microalgae cells, Pi transporters on the cell membrane promote the uptake of phosphoric acid so that Pi can be uptake from the external environment into the cell. Inside the cell, phosphorus is used in various metabolic pathways, including the synthesis of nucleic acids (e.g., DNA, RNA) and lipid membranes (e.g., phospholipids). Moreover, phosphorus is essential for the synthesis of adenosine triphosphate (ATP), which is the main energy currency in cells [62]. ATP is synthesized through oxidative phosphorylation or photophosphorylation and then hydrolyzed to adenosine diphosphate (ADP) and Pi, releasing energy. This energy transfer, known as the ATP–ADP cycle, provides power to various cellular processes, including protein production [63]. Overall, optimizing pre-treatment improves nutrient bioavailability and enhances metabolic activity of microalgae. Effective integration of pre-treatment methods with microalgal nutrient metabolism not only promotes efficient food waste valorization, but also addresses nutrient pollution and environmental sustainability.
Strain selection, growth modes, and culture conditions of microalgae using food waste hydrolysates
Food waste hydrolysates are generated through pre-treatment and decomposition of organic waste materials in waste valorization [64]. Due to the variations in their composition, it is necessary to select microalgal strains that are compatible with specific nutrient profiles and cultivation conditions. Ideal strains should tolerate complex substrates, exhibit rapid growth, and accumulate high lipid content. Chlorella sorokiniana achieved robust heterotrophic growth at 32 ℃ by utilizing enzymatically hydrolyzed municipal food waste rich in glucose and acetic acid. These substrates, along with dark and oxygenated culture conditions, supported efficient carbon assimilation without photosynthesis [65]. This metabolic mechanism circumvented photoinhibition and enabled biomass increase under elevated temperatures. Moreover, Coelastrella saipanensis demonstrated sustained growth at 27 °C under PSII-inhibited conditions induced by DCMU, reflecting its ability to shift to heterotrophy and assimilate organic nutrients from dairy wastewater [66]. The microalgal strain thermotolerance and mixotrophic flexibility enabled survival without photosynthetic carbon fixation.
Overall, the variations in algal response to different food waste substrates can be explained by differences in substrate composition, cultivation mode, and species-specific metabolic traits. Substrates containing readily assimilable organic carbon compounds generally support higher biomass and lipid yields, particularly under heterotrophic or mixotrophic conditions. In contrast, substrates that were poorly hydrolyzed or nutritionally imbalanced tended to limit microalgae growth. Furthermore, algal strains differed in thermal tolerance and carbon assimilation pathways, which influenced their performance under varying temperature and light regimes. These interacting factors jointly shaped the physiological and biochemical outcomes observed across the studies summarized in Table 1.
Phaeodactylum tricornutum ALE-Pt1 is a genetically adapted strain that was cultivated in food waste hydrolysates under mixotrophic conditions to produce PUFAs. This modified strain yielded 7.8 g biomass and 0.87 g EPA when grown with food waste hydrolysates supplemented with purified glucose [72]. This finding highlights how strain engineering and carbon source standardization can be combined to optimize target compound production. The metabolic mode of cultivation plays a determining role in how microalgae utilize substrates from food waste. Each growth mode alters metabolic fluxes, thereby influencing the quantity and quality of target products such as lipids, pigments, and proteins. For example, heterotrophic growth often favors lipid accumulation due to excess carbon availability, while photoautotrophic growth enhances pigment (e.g., chlorophyll, carotenoids) biosynthesis due to light-driven photosynthetic pathways. Mixotrophic growth can lead to a balanced production of biomass, lipids, and pigments by integrating both autotrophic and heterotrophic metabolic routes. Protein content is often maintained at higher levels under photoautotrophic and mixotrophic conditions, depending on nitrogen availability and stress factors [73]. Therefore, the choice of growth mode should be aligned with the desired product, whether it is biomass, lipids, pigments, or proteins.
A study conducted by Cheng et al. (2022) found that both heterotrophic and mixotrophic cultivation improved lipid content compared to photoautotrophic conditions, with heterotrophy reaching 18.53% total lipids [74]. Moreover, cultivation parameters such as temperature, pH, light regime, and nutrient availability (especially nitrogen and phosphorus) directly influence lipid metabolism in microalgae. Specifically, nutrient stress (e.g., nitrogen or phosphorus limitation) and modified light conditions (e.g., changes in intensity, photoperiod, or spectral composition) have been shown to enhance lipid accumulation and alter fatty acid profiles [75]. To further enhance productivity, two-stage cultivation is often applied. In the first stage, optimal conditions promote biomass accumulation, and in the second stage, stress conditions (e.g., nitrogen limitation) are introduced to stimulate lipid biosynthesis [76]. This approach decouples growth and lipid accumulation, increasing the overall yield of valuable products. In summary, the efficient use of food waste hydrolysates for lipid production depends on selecting compatible microalgal strains, employing heterotrophic or mixotrophic growth modes, and designing cultivation strategies tailored to the metabolic and environmental constraints of both the microalgae and the waste substrate.
Upcycling strategies for food waste toward sustainable microalgae-derived lipid production
Repurposing food waste into alternative culture media for microalgae cultivation offers a sustainable route for lipid production, particularly for high-value functional lipids such as PUFAs. This approach directly addresses food waste and resource inefficiency while reducing the cost of microalgae cultivation [12]. Pre-treated food waste increases the bioavailability of key nutrients (e.g., carbon, nitrogen, phosphorus), thereby enhancing microalgae growth and lipid accumulation. These advantages underscore the importance of optimizing food waste pre-treatments not only to maximize nutrient recovery, but also to improve downstream lipid extraction efficiency. Both solid and liquid food waste streams can be converted into culture media for microalgae cultivation within a circular bioeconomy framework. This integrated approach transforms waste into valuable bioproducts and highlights the dual benefit of environmental management and economic feasibility [77].
Integrated framework for sustainable food waste valorization through microalgae-derived lipid production
An integrated framework that repurposes food waste as nutrient source for microalgae cultivation and enables lipid extraction from the resulting biomass contributes directly to the core objectives of the circular bioeconomy. This framework consists of several interconnected stages: collection of food waste, food waste pre-treatment, microalgae cultivation, lipid extraction, and application of extracted lipids in sectors such as biofuels, nutritional supplements (e.g., omega-3 and omega-6 fatty acids), and cosmetics [78]. A recent study developed an integrated biorefinery approach in which pre-treated food waste hydrolysate was used to cultivate Chlorella vulgaris, while the residual solids were directed to biomethane recovery. When hydrolysates with a solubility of 40–45% were used, microalgal biomass productivity reached 0.87 ± 0.003 g per g substrate, and lipid accumulation was 360 ± 15 mg per g substrate [79].
Figure 3 illustrates the stepwise process of converting food waste into hydrolysates suitable for microalgae cultivation and subsequent lipid production in a circular bioeconomy. The first step includes the assessment and collection of food waste from households, the food service industry, and food processing facilities. It is necessary to separate organic from inorganic materials to ensure the quality of the feedstock [80]. Efficient collection systems across different sectors are required to transport food waste to processing sites, ensuring a steady supply of feedstock for producing a consistent and nutrient-rich culture medium. In the pre-treatment stage, solid food waste undergoes physical disruption (e.g., grinding, crushing), followed by microbial inoculation for solid-state fermentation (SSF). During SSF, microorganisms (e.g., fungi or bacteria) degrade complex organic matter into simpler compounds through enzymatic activity, thereby enhancing the nutrient content and bioavailability in the culture medium [81, 82].
On the other hand, liquid food waste is often used more directly, although it often requires pH adjustment and the removal of potential pollutants [10]. Due to its high moisture content and readily available soluble nutrients, liquid food waste is well suited for heterotrophic and mixotrophic cultivation modes, both of which rely on organic carbon sources rather than light [65, 68]. Recent studies have shown that when nutrient-rich substrates such as hydrolyzed canteen food waste are used, optimization of these cultivation strategies can significantly enhance microalgal lipid productivity. For example, Nannochloropsis oceanica cultivated under mixotrophic conditions using hydrolyzed canteen food waste demonstrated a significant increase in PUFAs, with EPA accounting for up to 32% of total FAMEs [83]. In addition, a study aimed to identify the optimal microalgal species for cultivation on digestate derived from organic solid waste. It also evaluated the effects of different digestate pre-treatment methods on algal growth and nutrient uptake. Chlorella vulgaris demonstrated superior growth performance in the digestate compared to Scenedesmus obliquus. Among the pre-treatment methods, centrifugation alone was sufficient to meet the growth requirements of microalgae, without the need for additional filtration steps [84]. This finding indicates that simplified pre-treatment can reduce operational costs and enhance the feasibility of microalgae-based waste valorization within the framework of the circular bioeconomy.
After cultivation, microalgal biomass is harvested by techniques such as centrifugation or filtration. Lipid extraction methods such as solvent extraction, supercritical fluid extraction, and enzymatic extraction are then applied. The extracted lipids are purified and tailored for targeted applications in biofuels or as feedstocks for food and cosmetic industries [85, 86]. In addition to lipid extraction, the residual microalgal biomass can be repurposed as a soil enhancer, contributing to the circular bioeconomy by returning nutrients to the agricultural cycle [87]. Table 2 evaluates the key process stages, associated benefits, and technical considerations in the valorization of food waste through microalgae cultivation for lipid production. In conclusion, this integrated framework demonstrates the potential of food waste upcycling through microalgae cultivation to generate added value while mitigating key environmental issues.
Upcycling liquid food waste streams for enhanced microalgae-derived lipid production
Liquid food waste, originating from various sources such as households, restaurants, and food manufacturing factories, contains high concentrations of organic matter, including fats, oils, and greases (FOG) [88]. However, it is also characterized by high levels of biochemical oxygen demand (BOD), chemical oxygen demand (COD), total dissolved solids (TDS), total suspended solids (TSS), total nitrogen (TN), and phosphate (TP), which can cause environmental issues if not properly treated [89]. These characteristics make liquid food waste a promising feedstock for microalgae cultivation, provided it is properly characterized and conditioned. Several studies have underscored the feasibility and effectiveness of using microalgae to treat liquid food waste while simultaneously producing lipids. For example, Kam et al. (2017) reported a lipid productivity of 244.2 mg/L/day when cultivating microalgae using starch-rich brewery effluent [90]. In another study, three types of liquid food waste including brewery wastewater, cheese whey, and expired orange juice were investigated as alternative media for microalgae cultivation [91]. Chlorella vulgaris CCAP 211/11B, Nannochloropsis oculata CCAP 849/1 and Scenedesmus sp. CCAP 276/41 were cultured in these waste streams. Among the tested substrates, expired orange juice significantly enhanced microalgal growth due to its high carbohydrate content and favorable pH conditions. Chlorella vulgaris demonstrated the highest biomass productivity and lipid accumulation potential. Furthermore, all cultivated microalgae achieved high nutrient removal efficiencies, with reductions in TN, TP and COD exceeding 80–90%. Additionally, Almutairi et al. (2021) demonstrated that pre-treating lipidic food waste followed by microalgae cultivation yielded fatty acid methyl esters (FAMEs) at a recovery rate of 92.6% based on lipid content [92]. Overall, these results underscore the potential of upcycling liquid food waste streams for both environmental remediation and the production of lipid-rich microalgal biomass.
Upcycling solid food waste via solid-state fermentation for valuable microalgae-derived lipid production
Solid-state fermentation (SSF) provides an effective method to upcycle solid food waste into nutrient-rich media for microalgae cultivation. Unlike liquid fermentation processes such as submerged fermentation (SmF), SSF involves the growth of microorganisms on moist, non-soluble solid substrates with little or no free water [93]. This approach facilitates the enzymatic degradation of complex compounds by microorganisms, producing bioavailable nutrients suitable for microalgae cultivation [94]. Therefore, these key parameters such as substrate type, moisture content, temperature, aeration, inoculum size, and pH must be optimized to maximize bioconversion efficiency in SSF performance [95]. For instance, using fruit and vegetable waste as substrates in SSF has been shown to increase the protein content by up to 1.9 times compared to untreated material [96], resulting in generating a nitrogen-rich fermentation product. When this product is used as a culture medium for microalgae cultivation, it promotes microalgal biomass accumulation and improves the metabolic adaptability necessary for lipid biosynthesis. Moisture content is another crucial factor in SSF because insufficient moisture reduces nutrient diffusion, microbial growth, enzyme stability, and substrate swelling [82]. Generally, the moisture content for SSF ranges from 30 to 80%, depending on the type of substrate used, the microbial strain involved, and the specific requirements of the fermentation process [97]. Temperature is another key factor that directly affects the rate of fermentation and microbial activity in SSF. Fungi used in SSF can grow over a broad temperature range from 20 to 55 °C. However, the optimal temperature varies depending on the species and fermentation conditions. In many cases, mesophilic strains are employed, with optimal performance observed at temperatures not exceeding 50 °C [98].
Inoculum size also plays a critical role microbial colonization and metabolic efficiency. For example, in SSF food waste by Aspergillus tubingensis, an inoculum size of 10% (w/w) yielded significantly higher biomass and enzyme production than 5%, while inoculation above 15% led to nutrient depletion and lower productivity [99]. This study also confirmed increased carbohydrate release, which benefits downstream microalgae cultivation. Moreover, organic carbon compounds released from solid food waste under SSF conditions can be assimilated by microalgae, triggering higher lipid synthesis through heterotrophic growth [100]. Building on this principle, recent studies have underscored the feasibility of integrating SSF-derived nutrients with microalgal cultivation for high-value lipid production. For instance, Bhattacharya et al. (2023) reported the extraction of EPA-rich oil from Nannochloropsis sp. utilizing low-cost cellulolytic enzymes in an SSF bioreactor [94]. Their results showed a maximum total fatty acid (TFA) recovery of 369.4 ± 4.6 mg/g dry weight, corresponding to a TFA yield of 77%, with EPA comprising 11% of the extracted oil. These findings confirm the potential of SSF as a platform for efficient microalgae-derived lipid production. In summary, SSF provides a viable pathway to convert solid food waste into value-added culture media for microalgae cultivation and lipid accumulation. It aligns well with circular bioeconomy principles by closing nutrient loops, reducing waste, and generating high-value lipids. Future research should focus on scaling up SSF systems, developing robust microbial consortia for consistent performance, and integrating SSF with other bioprocesses (e.g., anaerobic digestion, hydrothermal carbonization) to improve the resource-use efficiency of lipid production platforms. Figure 4 illustrates the integrated schematic of SSF and SmF processes pre-treated solid food waste through microalgae cultivation.
Schematic overview of solid-state (SSF) and submerged (SmF) fermentation pathways for microalgae cultivation from pre-treated solid food waste
Microalgae-derived lipid production to achieve net-zero food waste emissions and transitioning toward a circular bioeconomy
The growing demand for fish oils, predominantly extracted from overexploited small pelagic fish (e.g., anchovies and sardines), is exacerbating marine biodiversity loss and trophic cascade disruptions [101, 102]. Lipids synthesized from microalgal biomass offer a viable alternative, with species such as Schizochytrium sp. producing lipid profiles containing 31.4% DHA and 3.5% EPA, comparable to conventional fish oil concentrations [103]. Importantly, using food waste streams to cultivate these oleaginous microalgae contributes directly to net-zero food waste emissions by diverting organic residues from conventional disposal pathways such as landfilling and incineration, which are typically associated with GHG emissions and nutrient loss. This valorization facilitates the reuse of organic matter and nutrients to produce eco-friendly and value-added bioproducts such as biodiesel, bioethanol, omega-3-rich oils, protein-rich animal feed, and biofertilizers [10, 104], thereby closing nutrient and carbon loops in alignment with the goals of a circular bioeconomy. However, scalability constraints persist, as traditional open-pond systems exhibit low lipid productivity, necessitating energy-intensive photobioreactors (PBRs) to reach industrial-scale outputs. Economic viability is further challenged by production costs and environmental trade-offs associated with nutrient input, energy consumption, and harvesting techniques.
Microalgae-derived lipids as sustainable alternatives to fish oil for omega-3 fatty acid production
The increasing global demand for omega-3 PUFAs (e.g., EPA, DHA) has traditionally been met by extracting oils from marine fish. However, the continued exploitation of small pelagic fish through unsustainable practices such as bottom trawling not only threatens marine biodiversity but also contributes to carbon release and ecosystem destabilization [105, 106]. In contrast, microalgae naturally produce omega-3-rich lipids and represent a promising and ecologically viable substitute for fish-derived oils [107]. Microalgae-derived lipids are structurally identical to those from fish oil, ensuring equivalent bioavailability and health benefits [108]. Feeding trials have confirmed the nutritional adequacy of these algal oils, as demonstrated by the successful replacement of fish oil in aquaculture diets without affecting the survival, growth, or feed efficiency of gilthead sea bream fingerlings [109]. In addition, oleaginous microalgae can accumulate lipid concentrations exceeding 45% of their dry cell weight under environmentally stressful conditions [103]. Moreover, the use of food waste as a carbon source not only supports lipid accumulation, but also reduces the environmental footprint of cultivation, directly contributing to the mitigation of food system emissions. For instance, mixotrophic cultivation of evolved Nannochloropsis oceanica utilizing food waste hydrolysate resulted in biomass productivity and lipid accumulation of 49% of dry cell weight and 32% of total FAMEs, respectively [83].
Scalability considerations for microalgae-derived lipid production
Scalable production of microalgae-derived lipids requires addressing biological and technological bottlenecks across upstream and downstream processes. One recent approach to upstream optimization involves combining adaptive laboratory evolution (ALE) with food waste upcycling to improve lipid accumulation efficiency. Pugazhendhi and Sharma (2024) demonstrated this dual strategy by evolving Nannochloropsis oceanica strains under hyposalinity stress. The improved strains (ALE2) exhibited robust growth and achieved a lipid content of 45% of cell dry weight when cultivated on food waste hydrolysate, representing a 2.3-fold increase compared to the wild-type strains [83]. The choice of cultivation system is critical for large-scale microalgal lipid production because it affects biomass productivity, operational cost, environmental impact, and scalability. Microalgae cultivation systems are mainly divided into open raceway ponds (ORPs) and closed photobioreactors (PBRs). ORPs are cost-effective due to their relatively low capital and operational expenditures, and their simple construction and maintenance make them suitable for large-scale cultivation. However, ORPs currently face several bottlenecks, including low biomass concentration, susceptibility to contamination, and high energy consumption [110]. In contrast, PBRs are utilized for cultivating microalgae under controlled conditions, which improve light availability, gas exchange, temperature, and nutrient distribution, thereby increasing biomass productivity and reducing contamination risks compared to ORPs [111].
Furthermore, innovations such as integrating flue gas and wastewater into microalgae cultivation system can reduce costs and improve sustainability. For instance, a study deployed 12–15 airlift photobioreactors (AL-PBRs) in a closed-loop biorefinery system that recycled wastewater rich in glycerol (4–15 g/L) and captured CO₂ from flue gas (12–20% mol), achieving 1.05 g/L Chlorella vulgaris biomass concentration. By maintaining optimal flow regimes and light/dark cycles, the system produced 228 L/day of biodiesel while reusing 28.8 L/day of wastewater and 90 m3/h of flue gas, achieving net-zero emissions and enabling waste-to-energy conversion with 9–9.5 kW power generation [112]. In addition, pre-acclimation of Micractinium pusillum KMC8 to dissolved flue gas compounds such as NOx and SOx enhanced CO2 fixation (136.79 mg/L/ d), lipid accumulation (32%), and biomass yield (1.32 g/L), demonstrating that toxic flue gas can serve as both a carbon and nutrient source for cost-effective and sustainable microalgae cultivation [113]. Building on these upstream advancements, downstream integration further supports scalability through biorefinery systems that extract lipids for biofuels and high-value omega-3 fatty acids while valorizing residual biomass for applications such as animal feed, biofertilizers, bioplastics, and biochar [114, 115]. This integrated approach indicates that coupling upstream resource recovery with downstream biorefinery valorization enables a scalable, zero-waste microalgae-based bioeconomy.
Economic feasibility and environmental trade-offs for microalgae-derived lipid production
The transition to microalgae-derived lipids as fish oil substitutes must be evaluated in terms of both economic feasibility and environmental impacts. At present, the cost of microalgae cultivation and lipid extraction remains a significant barrier due to energy-intensive operations, particularly in photobioreactor (PBR) systems. These economic challenges are compounded by environmental burdens stemming from high nutrient inputs, water consumption, and biomass harvesting processes. Integration of food waste as nutrient feedstock addresses both cost and carbon burdens, effectively enabling net-zero food waste emissions by diverting waste streams into productive bioresource pathways [116]. A techno-economic analysis (TEA) of Aurantiochytrium sp. cultivation for DHA production showed that replacing conventional nutrient sources with food waste streams (e.g., brewery by-products, dairy wastewater) reduced raw material costs tenfold and unit production costs by up to 38%, while doubling the net present value (NPV) and improving return on investment (ROI) by 8% [117]. Similarly, Japonochytrium marinum AN-4 grown in saline dairy wastewater obtained DHA productivity of 0.86 g/L/day at a medium cost of $38.9–40 per m3, which is less than half the cost of a conventional medium ($71.4 per m3) [118].
From an environmental trade-off perspective, a life cycle assessment (LCA) conducted by Bartek et al. (2021) indicated that producing DHA from microalgae cultivated on food waste resulted in significantly lower global warming potential, eutrophication, and land use compared to traditional fish oil production [119]. Therefore, converting food waste into a nutrient source for microalgae cultivation and subsequently extracting lipids from the harvested biomass reduces GHG emissions and contributes to net-zero food waste emissions. Nevertheless, traditional solvent extraction techniques (e.g., chloroform and methanol) present toxicity concerns in omega-3 fatty acids production [120]. To address this issue, green extraction technologies such as deep eutectic solvents (DES) and supercritical fluid extraction (SFE) are being employed. A recent study using choline chloride-ethylene glycol DES obtained improved extraction efficiency for fatty acids from Nannochloropsis gaditana biomass, particularly enhancing the recovery of omega-3 EPA. By applying ultrasonic treatment combined with NaCl, the study achieved over 18% higher EPA recovery compared to the HCl–methanol extraction method [121]. Likewise, SFE utilizes supercritical CO2 as a green solvent, eliminating reliance on synthetic chemicals [122]. This technique treats microalgal biomass under precisely controlled temperature and pressure conditions to improve lipid recovery [123]. Mathur et al. (2022) reported that SFE extracted 3.0 ± 0.4% total lipids, which was lower than the 28.2 ± 2.3% obtained by Soxhlet extraction using a 1:1 (v/v) chloroform–methanol mixture. However, the lipids extracted by SFE contained a significantly higher proportion of PUFAs at 20.68%, compared to only 3.48% in those obtained by the Soxhlet method [14]. Overall, these findings suggest that DES and SFE are promising lipid extraction methods that not only minimize environmental impacts but also improve the nutritional profiles of microalgae-derived lipids. Figure 5(a) compares the EPA and DHA contents, expressed as percentages of total fatty acids, in selected microalgal species and commercial fish oil capsules. Nannochloropsis sp. and Thalassiosira pseudonana show high EPA contents comparable to those in Swisse Ultiboost odorless wild fish oil, but contain negligible DHA. In contrast, Schizochytrium sp. contains nearly 50% DHA, similar to the composition of Blackmores omega brain capsules, highlighting its potential as a DHA-rich microalgal source.
Technical barriers and strategic pathways for scaling microalgae-derived lipid production from food waste
Even though significant progress has been made in using food waste as substitute culture media for microalgae growth and to enhance lipid production, its contribution towards achieving net-zero food waste emissions in the global food system still faces several challenges due to economic, technological, environmental, social, and regulatory barriers. This section outlines practical implementation strategies, supported by case studies and pilot programs, and discusses key obstacles to commercialization.
Environmental, economic, social, and safety considerations for microalgae-derived lipid production using food waste
Using food waste to cultivate microalgae for lipid production represents an innovative strategy with the potential to transform sustainable resource recovery into waste management. This method not only diverts food waste from landfills and incinerators, but also contributes to the sustainable production of value-added lipids for use in biofuels, animal feed, and nutraceuticals [10]. However, several environmental concerns are specifically associated with the use of food waste as an alternative culture medium. For instance, liquid food waste often contains high levels of nutrients, and if not properly treated before microalgae cultivation, it may lead to excess nutrient loading in aquatic systems during storage or accidental discharge. This could trigger harmful algal blooms (HABs), resulting in oxygen depletion and biodiversity loss [126]. Additionally, the improper disposal of untreated or excess food waste remains a separate environmental threat, further contributing to GHG emissions and eutrophication. To mitigate these risks, strategies such as controlled nutrient release and real-time monitoring of nutrient concentrations in liquid food waste streams have been proposed [127]. Moreover, while microalgae cultivation using food waste can reduce reliance on synthetic media, it remains energy-intensive, particularly during biomass harvesting and lipid extraction [128]. To improve environmental performance, Huang et al. (2022) have emphasized the importance of using green solvents and integrated extraction methods that minimize energy input and maximize net energy ratio (NER). Their results showed an increase in NER to 0.73 and a reduction in GHG emissions to − 41.12 g CO2-eq MJ−1 [129], thereby advancing both economic and environmental performance.
From an economic standpoint, high operational expenditures (OPEX) and capital expenditures (CAPEX) have long been barriers to the commercial-scale adoption of microalgae-derived lipids. However, a TEA study demonstrated that the algal turf scrubber (ATS) growth system produces biomass at a cost of $510 per tonne, which is 39% lower than that of traditional open raceway pond (ORP) system ($673 per tonne). Despite both systems producing microalgae biofuel at $6.27 per gallon, the lower cost of ATS growth system highlights its economic viability for large-scale microalgae-derived lipids production [125]. Figure 5(b) illustrates the cost structure of both systems. The CAPEX of the ATS growth system ($339 per tonne) is comparable to that of the ORP growth system ($351 per tonne), the OPEX of ATS system is significantly lower ($118 per tonne) compared to the ORP system ($266 per tonne), contributing to the overall cost advantage of the ATS system. Therefore, this economic benefit positions the ATS system as a more viable option for large-scale applications and advances the commercialization of microalgae-derived lipids. Technological innovations, especially in upstream and downstream automation, can further reduce costs. Advanced photobioreactors (PBRs) integrated with Internet of Things (IoT) sensors have been developed to optimize real-time monitoring, nutrient delivery, and harvesting cycles [130]. As reported by Udayan et al. [12], smart bioreactor systems reduce labor needs and improve process control, leading to improved energy efficiency and increased lipid yields.
Social acceptance and consumer trust are also essential for successful commercialization. Functional products like omega-3-rich lipids and other beneficial compounds align with the interests of increasingly health-conscious consumers, driving rising demand for functional foods and supplements [131]. Transparency in sourcing, labeling, and safety validation, exemplified by Source-Omega company generally recognized as safe (GRAS) certified algae-based DHA supplement, can significantly enhance market confidence and accelerate product adoption. To ensure transparency and accountability, producers should adopt robust traceability systems and uphold clear and consistent communication strategies.
Addressing safety concerns associated with microalgae-derived lipid production is crucial to ensuring consumer confidence and regulatory compliance. Governments and industry stakeholders are increasingly convening multidisciplinary expert panels to establish guidelines for microalgae cultivation using food waste. These regulations should comprehensively address biosafety, environmental protection, product quality, traceability, and public engagement. Establishing standards for food-grade or feed-grade applications of microalgae-derived lipids will be key to reducing entry barriers in the market [132].
Research priorities and biotechnological challenges in developing microalgae-derived lipids as fish oil alternatives
The production of microalgae-derived lipids as sustainable substitutes for fish oil presents a promising pathway toward achieving net-zero emissions. Although research over the past decade has primarily focused on utilizing microalgal biomass for biofuel production, major challenges remain including infrastructure limitations, high energy intensity, and poor cost competitiveness [133]. Hence, it is crucial to shift toward exploring the potential of value-added bioproducts obtained from microalgae in terms of economic viability and environmental sustainability, which can have a substantial impact on reaching net-zero emissions. Key to this transition is the optimization of microalgae cultivation and lipid extraction processes to enhance efficiency and reduce costs [134]. Progress in genetic and metabolic engineering has shown potential in enhancing the efficiency and adaptability of microalgae strains. Engineered microalgae strains can be optimized to convert food waste into lipids more efficiently and to grow under specific environmental conditions, thus reducing dependence on energy-intensive cultivation resources [135]. For example, Ajiawi and teams demonstrated that suppressing the Zn2Cys6 transcriptional regulator in Nannochloropsis gaditana using CRISPR-Cas9 and RNA interference techniques doubled lipid production by enhancing carbon partitioning to lipids [136]. Similarly, Wang et al. (2024) emphasized the potential of genetic modification to enhance microalgal productivity and product quality when grown on food waste substrates. However, they also identified several persistent barriers, including incomplete understanding of metabolic pathways, low transformation efficiency, and biological contamination risks [56]. Despite promising lab-scale results, industrial scalability of microalgae-derived lipid production remains hindered by technical and regulatory barriers. Key challenges include inefficient genetic transformation systems and public apprehensions surrounding genetically modified organisms (GMOs) in food and feed applications [137]. Furthermore, genetically optimized microalgae strains exhibit trait drift when scaled up from laboratory to industrial scale [138], underscoring the need to develop resilient, adaptable microalgae capable of thriving in commercial-scale cultivation. Therefore, future efforts must prioritize interdisciplinary solutions that combine advanced genetic engineering, process optimization, and rigorous environmental testing. Addressing these gaps will be critical to advancing scalable, socially acceptable microalgae technologies that contribute meaningfully to net-zero emissions.
Moreover, the source and storage conditions of food waste also significantly affect the efficiency and sustainability of microalgae-based biorefinery. The composition of food waste, which differs according to its sources (e.g., household, agricultural, or industrial residues), influences the availability of nutrients such as carbohydrates and lipids essential for microalgae growth and metabolite synthesis [139]. For example, starch-rich waste can improve carbohydrate-to-lipid conversion, whereas protein-rich waste may increase nitrogen availability. Storage conditions also affect food waste quality: extended exposure to heat or moisture accelerates decomposition, fosters microbial competition, and induces acidification, hence diminishing nutritional bioavailability and producing inhibiting compounds such as volatile fatty acids [140, 141]. Therefore, it is important to regulate temperature, adjust pH to prevent premature fermentation, and manage moisture levels to limit bacterial contamination and preserve valuable organic compounds, with sterilization as needed. To bridge the gap between research and commercialization, future efforts must prioritize scalable solutions. This includes developing strains resilient to environmental fluctuations, refining genetic tools for higher transformation efficiency, and establishing policies that balance innovation with safety. Interdisciplinary collaboration among biologists, engineers, and policymakers will be essential to unlock the full potential of microalgae as a sustainable lipid source, ultimately advancing UN SDGs.
Conclusion
This study highlights the technical and environmental potential of converting food waste into a sustainable cultivation medium for microalgae to enhance lipid production. Replacing synthetic culture media with food waste not only redirects organic matter from landfills and incineration but also supports the circular bioeconomy by generating high-value lipids for biofuels, animal feed, and nutraceuticals. Key findings show that food waste hydrolysates can support robust microalgal growth and significantly enhance lipid accumulation, especially when the carbon-to-nitrogen-to-phosphorus (C/N/P) ratio is optimized. However, feedstock variability remains a major limitation, necessitating standardized pre-treatment methods to ensure consistent nutrient composition. Furthermore, technological advances such as AI-based predictive models and digital twins provide new approaches to optimize key process parameters and improve lipid yield. Nevertheless, scale-up is hindered by energy-intensive harvesting and lipid extraction, requiring integrated biorefinery designs and low-energy solutions to ensure economic viability. Although lab-scale feasibility is established, large-scale implementation still faces cost, contamination, and regulatory challenges. Future efforts should emphasize microalgae strain engineering for enhanced lipid biosynthesis, automation for continuous cultivation, and LCA to confirm environmental benefits. In summary, valorizing food waste for microalgae cultivation, followed by lipid extraction from the harvested biomass, provides a viable path toward net-zero emissions and aligns with several UN SDGs, including responsible consumption and production (SDG 12) and climate action (SDG 13), by coupling waste valorization with sustainable biofuel and functional lipid synthesis.
Data availability
No datasets were generated or analyzed during the current study.
Abbreviations
- FLW:
-
Food loss and waste
- GHG:
-
Greenhouse gas
- PUFA:
-
Polyunsaturated fatty acid
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- C/N/P:
-
Carbon-to-nitrogen-to-phosphorus
- LCA:
-
Life cycle assessment
- SDG:
-
Sustainable Development Goal
- FWM:
-
Food waste management
- CH4 :
-
Methane
- CO2 :
-
Carbon dioxide
- NOX :
-
Nitrogen oxides
- VOCs:
-
Volatile organic compounds
- sCOD:
-
Soluble chemical oxygen demand
- VS:
-
Volatile solids
- SCFAs:
-
Short-chain fatty acids
- VFAs:
-
Volatile fatty acids
- HMF:
-
Hydroxymethylfurfural
- ATP:
-
Adenosine triphosphate
- NADH:
-
Nicotinamide adenine dinucleotide
- FADH2 :
-
Flavin adenine dinucleotide
- GTP:
-
Guanosine triphosphate
- DHAP:
-
Dihydroxyacetone phosphate
- G3P:
-
Glyceraldehyde-3-phosphate
- RuBisCO:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- RuBP:
-
Ribulose-1,5-bisphosphate
- 3-PGA:
-
3-Phosphoglycerate acid
- GS/GOGAT:
-
Glutamine synthetase/glutamate synthase
- Pi:
-
Inorganic phosphate
- ADP:
-
Adenosine diphosphate
- SSF:
-
Solid-state fermentation
- SmF:
-
Submerged fermentation
- BOD:
-
Biochemical oxygen demand
- COD:
-
Chemical oxygen demand
- TDS:
-
Total dissolved solids
- TSS:
-
Total suspended solids
- FAMEs:
-
Fatty acid methyl esters
- ALE:
-
Adaptive laboratory evolution
- DES:
-
Deep eutectic solvents
- SFE:
-
Supercritical fluid extraction
- NPV:
-
Net present value
- ROI:
-
Return on investment
- CAPEX:
-
Capital expenditure
- OPEX:
-
Operating expenditure
- NER:
-
Net energy ratio
- TEA:
-
Techno-economic analysis
References
-
Food and Agriculture Organization. Global food losses and food waste—extent, causes and prevention. Rome, Italy: Food and Agriculture Organization; 2011.
-
UNEP. Food waste index report 2021. 2021.
-
FAO. The State of Food Security and Nutrition in the World 2023. FAO; IFAD; UNICEF; WFP; WHO; 2023.
-
Zhang H, Liu G, Xue L, Zuo J, Chen T, Vuppaladadiyam A, et al. Anaerobic digestion based waste-to-energy technologies can halve the climate impact of China’s fast-growing food waste by 2040. J Clean Prod. 2020;277: 123490.
-
Niemczyk M, Berenjkar P, Wilkinson N, Lozecznik S, Sparling R, Yuan Q. Enhancement of CH4 oxidation potential in bio-based landfill cover materials. Process Saf Environ Prot. 2021;146:943–51.
-
Sharma G, Sinha B. Future emissions of greenhouse gases, particulate matter and volatile organic compounds from municipal solid waste burning in India. Sci Total Environ. 2023;858:159708.
-
Li W, Yan D, Li L, Wen Z, Liu M, Lu S, et al. Review of thermal treatments for the degradation of dioxins in municipal solid waste incineration fly ash: proposing a suitable method for large-scale processing. Sci Total Environ. 2023;875: 162565.
-
Marques F, Pereira F, Machado L, Martins JT, Pereira RN, Costa MM, et al. Comparison of different pretreatment processes envisaging the potential use of food waste as microalgae substrate. Foods. 2024;13:1018.
-
Chan SS, Lee SY, Ling TC, Chae K-J, Srinuanpan S, Khoo KS. Unlocking the potential of food waste as a nutrient goldmine for microalgae cultivation: a review. J Clean Prod. 2025;492: 144753.
-
Kumar Y, Kaur S, Kheto A, Munshi M, Sarkar A, Om Pandey H, et al. Cultivation of microalgae on food waste: recent advances and way forward. Bioresour Technol. 2022;363: 127834.
-
Daneshvar E, Sik Ok Y, Tavakoli S, Sarkar B, Shaheen SM, Hong H, et al. Insights into upstream processing of microalgae: a review. Bioresour Technol. 2021;329: 124870.
-
Udayan A, Pandey AK, Sirohi R, Sreekumar N, Sang B-I, Sim SJ, et al. Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem Rev. 2023;22:833–60.
-
Malik S, Shahid A, Haider MN, Amin M, Betenbaugh MJ, Mehmood MA, et al. Prospects of multiproduct algal biorefineries involving cascading processing of the biomass employing a zero-waste approach. Curr Pollut Rep. 2022;8:147–58.
-
Mathur M, Hans N, Naaz F, Naik SN, Pant KK, Malik A. Valorization of microalgal biomass to value-added products using integrated supercritical CO2 extraction and sub-critical hydrothermal liquefaction. J Clean Prod. 2022;373: 133925.
-
Fatima I, Munir M, Qureshi R, Hanif U, Gulzar N, Sheikh AA. Advanced methods of algal pigments extraction: a review. Crit Rev Food Sci Nutr. 2024;64:9771–88.
-
Patel AK, Albarico FPJB, Perumal PK, Vadrale AP, Nian CT, Chau HTB, et al. Algae as an emerging source of bioactive pigments. Bioresour Technol. 2022;351: 126910.
-
Alishah Aratboni H, Rafiei N, Garcia-Granados R, Alemzadeh A, Morones-Ramírez JR. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb Cell Fact. 2019;18:178.
-
Cheirsilp B, Maneechote W, Srinuanpan S, Angelidaki I. Microalgae as tools for bio-circular-green economy: Zero-waste approaches for sustainable production and biorefineries of microalgal biomass. Bioresour Technol. 2023;387: 129620.
-
Olabi AG, Shehata N, Sayed ET, Rodriguez C, Anyanwu RC, Russell C, et al. Role of microalgae in achieving sustainable development goals and circular economy. Sci Total Environ. 2023;854: 158689.
-
Kumar V, Jaiswal KK, Tomar MS, Rajput V, Upadhyay S, Nanda M, et al. Production of high value-added biomolecules by microalgae cultivation in wastewater from anaerobic digestates of food waste: a review. Biomass Convers Biorefin. 2021;13:6925.
-
Deng Y, Chen F, Liao K, Xiao Y, Chen S, Lu Q, et al. Microalgae for nutrient recycling from food waste to aquaculture as feed substitute: a promising pathway to eco-friendly development. J Chem Technol Biotechnol. 2021;96:2496–508.
-
Chakraborty B, Gayen K, Bhowmick TK. Transition from synthetic to alternative media for microalgae cultivation: a critical review. Sci Total Environ. 2023;897: 165412.
-
Parthiba Karthikeyan O, Trably E, Mehariya S, Bernet N, Wong JWC, Carrere H. Pretreatment of food waste for methane and hydrogen recovery: a review. Bioresour Technol. 2018;249:1025–39.
-
Rawindran H, Khoo KS, Satpati GG, Maity S, Chandran K, Lim JW, et al. Composition of carbohydrate, protein and lipid derived from microalgae using thermally pretreated solid waste. J Sci Food Agric. 2025;105:4672–9.
-
Gallego-García M, Moreno AD, Manzanares P, Negro MJ, Duque A. Recent advances on physical technologies for the pretreatment of food waste and lignocellulosic residues. Bioresour Technol. 2023;369: 128397.
-
Li X, Mettu S, Martin GJO, Ashokkumar M, Lin CSK. Ultrasonic pretreatment of food waste to accelerate enzymatic hydrolysis for glucose production. Ultrason Sonochem. 2019;53:77–82.
-
Liu N, Jiang J, Yan F, Gao Y, Meng Y, Aihemaiti A, et al. Enhancement of volatile fatty acid production and biogas yield from food waste following sonication pretreatment. J Environ Manage. 2018;217:797–804.
-
González-Camejo J, Aparicio S, Jiménez-Benítez A, Pachés M, Ruano MV, Borrás L, et al. Improving membrane photobioreactor performance by reducing light path: operating conditions and key performance indicators. Water Res. 2020;172: 115518.
-
Joshi SM, Gogate PR. Intensifying the biogas production from food waste using ultrasound: understanding into effect of operating parameters. Ultrason Sonochem. 2019;59: 104755.
-
Wu Y, Yao S, Narale BA, Shanmugam A, Mettu S, Ashokkumar M. Ultrasonic processing of food waste to generate value-added products. Foods. 2022;11:2035.
-
Chen H, Wang Q. Regulatory mechanisms of lipid biosynthesis in microalgae. Biol Rev. 2021;96:2373–91.
-
Tomás-Pejó E, González-Fernández C, Greses S, Kennes C, Otero-Logilde N, Veiga MC, et al. Production of short-chain fatty acids (SCFAs) as chemicals or substrates for microbes to obtain biochemicals. Biotechnol Biofuels Bioproducts. 2023;16:96.
-
Liu J, Zhao M, Lv C, Yue P. The effect of microwave pretreatment on anaerobic co-digestion of sludge and food waste: performance, kinetics and energy recovery. Environ Res. 2020;189: 109856.
-
Yue L, Cheng J, Tang S, An X, Hua J, Dong H, et al. Ultrasound and microwave pretreatments promote methane production potential and energy conversion during anaerobic digestion of lipid and food wastes. Energy. 2021;228: 120525.
-
Li H, Xu J, Mbugua Nyambura S, Wang J, Li C, Zhu X, et al. Food waste pyrolysis by traditional heating and microwave heating: a review. Fuel. 2022;324: 124574.
-
Rajesh Banu J, Merrylin J, Mohamed Usman TM, Yukesh Kannah R, Gunasekaran M, Kim S-H, et al. Impact of pretreatment on food waste for biohydrogen production: a review. Int J Hydrogen Energy. 2020;45:18211–25.
-
Ravindran R, Jaiswal AK. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour Technol. 2016;199:92–102.
-
Hafid HS, Nor’Aini AR, Mokhtar MN, Talib AT, Baharuddin AS, Umi Kalsom MS. Over production of fermentable sugar for bioethanol production from carbohydrate-rich Malaysian food waste via sequential acid-enzymatic hydrolysis pretreatment. Waste Manag. 2017;67:95–105.
-
Linyi C, Yujie Q, Buqing C, Chenglong W, Shaohong Z, Renglu C, et al. Enhancing degradation and biogas production during anaerobic digestion of food waste using alkali pretreatment. Environ Res. 2020;188: 109743.
-
Wu Y, Hu W, Zhu Z, Zheng X, Chen Y, Chen Y. Enhanced volatile fatty acid production from food waste fermentation via enzymatic pretreatment: new insights into the depolymerization and microbial traits. ACS ES&T Eng. 2023;3:26–35.
-
Yousuf A, Bastidas-Oyanedel J-R, Schmidt JE. Effect of total solid content and pretreatment on the production of lactic acid from mixed culture dark fermentation of food waste. Waste Manage. 2018;77:516–21.
-
Ding L, Cheng J, Qiao D, Yue L, Li Y-Y, Zhou J, et al. Investigating hydrothermal pretreatment of food waste for two-stage fermentative hydrogen and methane co-production. Bioresour Technol. 2017;241:491–9.
-
Ramandani AA, Sun Y-M, Lan JC-W, Chen W-H, Chang J-S, Rachmadona N, et al. Upcycling nutrients derived from food waste via microalgae cultivation: a review on impacts on cellular compounds, economy and environment analyses for achieving circular bioeconomy. Biochem Eng J. 2024;211:109454.
-
Zhang Y, Wang J-H, Zhang J-T, Chi Z-Y, Kong F-T, Zhang Q. The long overlooked microalgal nitrous oxide emission: characteristics, mechanisms, and influencing factors in microalgae-based wastewater treatment scenarios. Sci Total Environ. 2023;856: 159153.
-
Sun H, Zhao W, Mao X, Li Y, Wu T, Chen F. High-value biomass from microalgae production platforms: strategies and progress based on carbon metabolism and energy conversion. Biotechnol Biofuels. 2018;11:227.
-
Merrylin J, Kannah RY, Banu JR, Yeom IT. Production of organic acids and enzymes/biocatalysts from food waste. Amsterdam: Elsevier; 2020.
-
Kierans SJ, Taylor CT. Glycolysis: a multifaceted metabolic pathway and signaling hub. J Biol Chem. 2024;300: 107906.
-
Proietti Tocca G, Agostino V, Menin B, Tommasi T, Fino D, Di Caprio F. Mixotrophic and heterotrophic growth of microalgae using acetate from different production processes. Rev Environ Sci Biotechnol. 2024;23:93–132.
-
Chilakamarry CR, Sakinah AMM, Zularisam AW, Pandey A. Glycerol waste to value added products and its potential applications. Syst Microbiol Biomanufact. 2021;1:378–96.
-
Ighalo JO, Dulta K, Kurniawan SB, Omoarukhe FO, Ewuzie U, Eshiemogie SO, et al. Progress in microalgae application for CO2 Sequestration. Clean Chem Eng. 2022;3: 100044.
-
Queiroz MI, Vieira JG, Maroneze MM. Morphophysiological, structural, and metabolic aspects of microalgae. Amsterdam: Elsevier; 2020.
-
Latagan MJD, Nagarajan D, Huang W-M, de Luna MDG, Chen J-H, Rollon AP, et al. Bicarbonate-based microalgal cultivation technologies: mechanisms, critical strategies, and future perspectives. Chem Eng J. 2024;502: 157998.
-
Yao D, Wu L, Tan D, Yu Y, Jiang Q, Wu Y, et al. Enhancing CO2 fixation by microalgae in a Photobioreactor: Molecular mechanisms with exogenous carbonic anhydrase. Bioresour Technol. 2024;408: 131176.
-
Dahai H, Zhihong Y, Lin Q, Yuhong L, Lei T, Jiang L, et al. The application of magical microalgae in carbon sequestration and emission reduction: removal mechanisms and potential analysis. Renew Sustain Energy Rev. 2024;197: 114417.
-
Yang B, Liu J, Ma X, Guo B, Liu B, Wu T, et al. Genetic engineering of the Calvin cycle toward enhanced photosynthetic CO2 fixation in microalgae. Biotechnol Biofuels. 2017;10:229.
-
Wang J, Wang Y, Xiao M, Liang Q, Yang S, Liu J, et al. Upcycling food waste into biorefinery production by microalgae. Chem Eng J. 2024;484: 149532.
-
Sanz-Luque E, Chamizo-Ampudia A, Llamas A, Galvan A, Fernandez E. Understanding nitrate assimilation and its regulation in microalgae. Front Plant Sci. 2015;6:899.
-
Kumar A, Bera S. Revisiting nitrogen utilization in algae: a review on the process of regulation and assimilation. Bioresour Technol Rep. 2020;12: 100584.
-
Tang W, Guo H, Baskin CC, Xiong W, Yang C, Li Z, et al. Effect of light intensity on morphology, photosynthesis and carbon metabolism of Alfalfa (Medicago sativa) Seedlings. Plants. 2022;11:1688.
-
Lu S, Chu G, Gao C, Zhao Y, Chen W, Jin C, et al. Effect of light intensity on nitrogen transformation, enzymatic activity, antioxidant system and transcriptional response of Chlorella pyrenoidosa during treating mariculture wastewater. Bioresour Technol. 2024;397: 130465.
-
Dyhrman ST. Nutrients and their acquisition: phosphorus physiology in microalgae. Cham: Springer International Publishing; 2016.
-
Wu Q, Guo L, Wang Y, Zhao Y, Jin C, Gao M, et al. Phosphorus uptake, distribution and transformation with Chlorella vulgaris under different trophic modes. Chemosphere. 2021;285: 131366.
-
Da Poian AT, Castanho MARB. Energy conservation in metabolism: the mechanisms of ATP synthesis. Cham: Springer International Publishing; 2021.
-
Roy P, Mohanty AK, Dick P, Misra M. A review on the challenges and choices for food waste valorization: environmental and economic impacts. ACS Environmental Au. 2023;3:58–75.
-
Haske-Cornelius O, Vu T, Schmiedhofer C, Vielnascher R, Dielacher M, Sachs V, et al. Cultivation of heterotrophic algae on enzymatically hydrolyzed municipal food waste. Algal Res. 2020;50: 101993.
-
Nayana K, Babu VS, Vidya D, Sudhakar MP, Arunkumar K. Growth and productivity of Haematococcus pluvialis and Coelastrella saipanensis by photosystem modulation for understanding the heterotrophic nutritional strategy for bioremediation application. Environ Res. 2024;245: 118077.
-
León-Vaz A, León R, Díaz-Santos E, Vigara J, Raposo S. Using agro-industrial wastes for mixotrophic growth and lipids production by the green microalga Chlorella sorokiniana. N Biotechnol. 2019;51:31–8.
-
Wang X, Zhang M-M, Sun Z, Liu S-F, Qin Z-H, Mou J-H, et al. Sustainable lipid and lutein production from Chlorella mixotrophic fermentation by food waste hydrolysate. J Hazard Mater. 2020;400: 123258.
-
Patel A, Rova U, Christakopoulos P, Matsakas L. Mining of squalene as a value-added byproduct from DHA producing marine thraustochytrid cultivated on food waste hydrolysate. Sci Total Environ. 2020;736: 139691.
-
Wang X, Zhang M-M, Liu S-F, Xu R-L, Mou J-H, Qin Z-H, et al. Synergistic bioconversion of lipids and carotenoids from food waste by Dunaliella salina with fulvic acid via a two-stage cultivation strategy. Energy Convers Manag. 2021;234: 113908.
-
Yang Y-F, Ye G-B, Wang H-J, Li H-Y, Lin CSK, Zheng X-F, et al. Utilization of lipidic food waste as low-cost nutrients for enhancing the potentiality of biofuel production from engineered diatom under temperature variations. Bioresour Technol. 2023;387: 129611.
-
Wang X, Liu S-F, Wang Z-Y, Hao T-B, Balamurugan S, Li D-W, et al. A waste upcycling loop: Two-factor adaptive evolution of microalgae to increase polyunsaturated fatty acid production using food waste. J Clean Prod. 2022;331: 130018.
-
Abreu AP, Morais RC, Teixeira JA, Nunes J. A comparison between microalgal autotrophic growth and metabolite accumulation with heterotrophic, mixotrophic and photoheterotrophic cultivation modes. Renew Sustain Energy Rev. 2022;159: 112247.
-
Cheng P, Huang J, Song X, Yao T, Jiang J, Zhou C, et al. Heterotrophic and mixotrophic cultivation of microalgae to simultaneously achieve furfural wastewater treatment and lipid production. Bioresour Technol. 2022;349: 126888.
-
Nzayisenga JC, Farge X, Groll SL, Sellstedt A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol Biofuels. 2020;13:4.
-
Aziz MMA, Kassim KA, Shokravi Z, Jakarni FM, Lieu HY, Zaini N, et al. Two-stage cultivation strategy for simultaneous increases in growth rate and lipid content of microalgae: a review. Renew Sustain Energy Rev. 2020;119: 109621.
-
Paini J, Benedetti V, Ail SS, Castaldi MJ, Baratieri M, Patuzzi F. Valorization of wastes from the food production industry: a review towards an integrated agri-food processing biorefinery. Waste Biomass Valorization. 2022;13:31–50.
-
Khan MI, Shin JH, Kim JD. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact. 2018;17:36.
-
Kavitha S, Ravi YK, Kumar G, Tyagi VK, Rajesh BJ. Integrated microalgae cultivation and sustainable biofuel production from pretreated food waste. J Clean Prod. 2024;466: 142829.
-
Molina-Peñate E, Artola A, Sánchez A. Organic municipal waste as feedstock for biorefineries: bioconversion technologies integration and challenges. Rev Environ Sci Biotechnol. 2022;21:247–67.
-
Cano y Postigo LO, Jacobo-Velázquez DA, Guajardo-Flores D, Garcia Amezquita LE, García-Cayuela T. Solid-state fermentation for enhancing the nutraceutical content of agrifood by-products: recent advances and its industrial feasibility. Food Biosci. 2021;41:100926.
-
Chilakamarry CR, Mimi Sakinah AM, Zularisam AW, Sirohi R, Khilji IA, Ahmad N, et al. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: opportunities and challenges. Bioresour Technol. 2022;343: 126065.
-
Pugazhendhi A, Sharma A. Food waste upcycling via microalgal adaptive evolution for lipids and fatty acids enhancement towards circular bioeconomy. Chem Eng J. 2024;484: 149593.
-
Scarponi P, Volpi Ghirardini AM, Bravi M, Cavinato C. Evaluation of Chlorella vulgaris and Scenedesmus obliquus growth on pretreated organic solid waste digestate. Waste Manag. 2021;19:235.
-
Severo IA, Siqueira SF, Deprá MC, Maroneze MM, Zepka LQ, Jacob-Lopes E. Biodiesel facilities: What can we address to make biorefineries commercially competitive? Renew Sustain Energy Rev. 2019;112:686–705.
-
Laurens LML, Markham J, Templeton DW, Christensen ED, Van Wychen S, Vadelius EW, et al. Development of algae biorefinery concepts for biofuels and bioproducts; a perspective on process-compatible products and their impact on cost-reduction. Energy Environ Sci. 2017;10:1716–38.
-
Vishwakarma R, Dhaka V, Ariyadasa TU, Malik A. Exploring algal technologies for a circular bio-based economy in rural sector. J Clean Prod. 2022;354: 131653.
-
Collin T, Cunningham R, Jefferson B, Villa R. Characterisation and energy assessment of fats, oils and greases (FOG) waste at catchment level. Waste Manage. 2020;103:399–406.
-
Asgharnejad H, Khorshidi Nazloo E, Madani Larijani M, Hajinajaf N, Rashidi H. Comprehensive review of water management and wastewater treatment in food processing industries in the framework of water-food-environment nexus. Compr Rev Food Sci Food Saf. 2021;20(5):4779–815.
-
Kam Y, Sung M, Cho H, Kang C-M, Kim J, Han J-I. Utilization of Starch-Enriched Brewery (Rice Wine) Waste for Mixotrophic Cultivation of Ettlia Sp. YC001 Used in Biodiesel Production. Appl Biochem Biotechnol. 2017;183:1478–87.
-
Anagnostopoulou C, Papachristou I, Kontogiannopoulos KN, Mourtzinos I, Kougias PG. Optimization of microalgae cultivation in food industry wastewater using microplates. Sustain Chem Pharm. 2024;39: 101510.
-
Almutairi AW, Al-Hasawi ZM, Abomohra A. Valorization of lipidic food waste for enhanced biodiesel recovery through two-step conversion: a novel microalgae-integrated approach. Bioresour Technol. 2021;342:125966.
-
Lee SY, Ra CH. Comparison of liquid and solid-state fermentation processes for the production of enzymes and beta-glucan from hulled barley. J Microbiol Biotechnol. 2022;32:317–23.
-
Bhattacharya R, Sachin S, Sivakumar R, Ghosh S. Solid-state fermentation-based enzyme-assisted extraction of eicosapentaenoic acid-rich oil from Nannochloropsis sp. Bioresour Technol. 2023;374: 128763.
-
Šelo G, Planinić M, Tišma M, Tomas S, Koceva Komlenić D, Bucić-Kojić A. A Comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods. 2021;10:927.
-
Ibarruri J, Cebrián M, Hernández I. Valorisation of fruit and vegetable discards by fungal submerged and solid-state fermentation for alternative feed ingredients production. J Environ Manage. 2021;281: 111901.
-
Muniz CES, Santiago ÂM, Gusmão TAS, Oliveira HML, de Conrado L. Solid-state fermentation for single-cell protein enrichment of guava and cashew by-products and inclusion on cereal bars. Biocatal Agric Biotechnol. 2020;25:101576.
-
Jiang Y, Xin F, Lu J, Dong W, Zhang W, Zhang M, et al. State of the art review of biofuels production from lignocellulose by thermophilic bacteria. Bioresour Technol. 2017;245:1498–506.
-
Manuel C-R, Carlos Q-F, Carmen P-C, Marisela V-DL, Iván M-A. Fungal solid-state fermentation of food waste for biohydrogen production by dark fermentation. Int J Hydrogen Energy. 2022;47:30062–73.
-
Kothri M, Mavrommati M, Elazzazy AM, Baeshen MN, Moussa TAA, Aggelis G. Microbial sources of polyunsaturated fatty acids (PUFAs) and the prospect of organic residues and wastes as growth media for PUFA-producing microorganisms. FEMS Microbiol Lett. 2020;5:028.
-
Gatto A, Sadik-Zada ER, Özbek S, Kieu H, Nguyen Huynh NT. Deep-sea fisheries as resilient bioeconomic systems for food and nutrition security and sustainable development. Resour Conserv Recycl. 2023;197: 106907.
-
Majluf P, Matthews K, Pauly D, Skerritt DJ, Palomares MLD. A review of the global use of fishmeal and fish oil and the Fish In: Fish Out metric. Sci Adv. 2024;10:5650.
-
Ma M, Hu Q. Microalgae as feed sources and feed additives for sustainable aquaculture: prospects and challenges. Rev Aquac. 2024;16:818–35.
-
Cheirsilp B, Maneechote W. Insight on zero waste approach for sustainable microalgae biorefinery: Sequential fractionation, conversion and applications for high-to-low value-added products. Bioresour Technol Rep. 2022;18:101003.
-
McConnaughey RA, Hiddink JG, Jennings S, Pitcher CR, Kaiser MJ, Suuronen P, et al. Choosing best practices for managing impacts of trawl fishing on seabed habitats and biota. Fish Fish. 2020;21:319–37.
-
Islam MZ. Ocean wildlife and Megafauna protection. Cham: Springer International Publishing; 2024.
-
Veerasamy V, Neethirajan V, Singarayar MS, Balasundaram D, Dharmar P, Thilagar S. Microalgal biomass and lipid synergy for omega fatty acid enrichment: a sustainable source for food supplements & nutraceuticals. Algal Res. 2024;80: 103514.
-
Barta DG, Coman V, Vodnar DC. Microalgae as sources of omega-3 polyunsaturated fatty acids: Biotechnological aspects. Algal Res. 2021;58: 102410.
-
Carvalho M, Montero D, Rosenlund G, Fontanillas R, Ginés R, Izquierdo M. Effective complete replacement of fish oil by combining poultry and microalgae oils in practical diets for gilthead sea bream (Sparus aurata) fingerlings. Aquaculture. 2020;529: 735696.
-
Xu Y, Wei C, Liu D, Li J, Tian B, Li Z, et al. Life-cycle and economic assessments of microalgae biogas production in suspension and biofilm cultivation systems. Bioresour Technol. 2024;395: 130381.
-
Pechsiri JS, Thomas J-BE, El BN, Fernandez FGA, Chaouki J, Chidami S, et al. Comparative life cycle assessment of conventional and novel microalgae production systems and environmental impact mitigation in urban-industrial symbiosis. Sci Total Environ. 2023;854:158445.
-
Paladino O, Neviani M. Airlift photo-bioreactors for Chlorella vulgaris cultivation in closed-loop zero waste biorefineries. Biomass Bioenergy. 2021;144: 105926.
-
Singh Chauhan D, Sahoo L, Mohanty K. Maximize microalgal carbon dioxide utilization and lipid productivity by using toxic flue gas compounds as nutrient source. Bioresour Technol. 2022;348: 126784.
-
Saini N, Dhull P, Manzoor I, Mushtaq B, Rao R. Utilization of Biomass to Produce Biofuels, Fertilizers, Biochar, and Other Value-Added Products. Singapore: Springer Nature Singapore; 2024.
-
Ezhumalai G, Arun M, Manavalan A, Rajkumar R, Heese K. A holistic approach to circular bioeconomy through the sustainable utilization of microalgal biomass for biofuel and other value-added products. Microb Ecol. 2024;87:61.
-
Diankristanti PA, Hei Ernest Ho N, Chen J-H, Nagarajan D, Chen C-Y, Hsieh Y-M, et al. Unlocking the potential of microalgae as sustainable bioresources from up to downstream processing: a critical review. Chem Eng J. 2024;488:151124.
-
Russo GL, Langellotti AL, Sacchi R, Masi P. Techno-economic assessment of DHA-rich Aurantiochytrium sp. production using food industry by-products and waste streams as alternative growth media. Bioresour Technol Rep. 2022;18:100997.
-
Humhal T, Kastanek P, Jezkova Z, Cadkova A, Kohoutkova J, Branyik T. Use of saline waste water from demineralization of cheese whey for cultivation of Schizochytrium limacinum PA-968 and Japonochytrium marinum AN-4. Bioprocess Biosyst Eng. 2017;40:395–402.
-
Bartek L, Strid I, Henryson K, Junne S, Rasi S, Eriksson M. Life cycle assessment of fish oil substitute produced by microalgae using food waste. Sustain Prod Consum. 2021;27:2002–21.
-
Swetha N, Mathanghi SK. Towards sustainable omega-3 fatty acids production—A comprehensive review on extraction methods, oxidative stability and bio-availability enhancement. Food Chem Adv. 2024;4: 100603.
-
Moreno Martínez P, Ortiz-Martínez VM, Sánchez Segado S, Salar-García MJ, et al. Deep eutectic solvents for the extraction of fatty acids from microalgae biomass: Recovery of omega-3 eicosapentaenoic acid. Sep Purif Technol. 2022;300:121842.
-
de Jesus SS, Filho RM. Recent advances in lipid extraction using green solvents. Renew Sustain Energy Rev. 2020;133: 110289.
-
Zhou J, Wang M, Saraiva JA, Martins AP, Pinto CA, Prieto MA, et al. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem. 2022;384: 132236.
-
Siddik MAB, Sørensen M, Islam SMM, Saha N, Rahman MA, Francis DS. Expanded utilisation of microalgae in global aquafeeds. Rev Aquac. 2024;16:6–33.
-
Hoffman J, Pate RC, Drennen T, Quinn JC. Techno-economic assessment of open microalgae production systems. Algal Res. 2017;23:51–7.
-
Singh K, Ansari FA, Ingle KN, Gupta SK, Ahirwal J, Dhyani S, et al. Microalgae from wastewaters to wastelands: Leveraging microalgal research conducive to achieve the UN Sustainable Development Goals. Renew Sustain Energy Rev. 2023;188: 113773.
-
Kibuye FA, Zamyadi A, Wert EC. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part I-chemical control methods. Harmful Algae. 2020;109.
-
Choudhary S, Tripathi S, Poluri KM. Microalgal-based bioenergy: strategies, prospects, and sustainability. Energy Fuels. 2022;24:14584.
-
Huang R, Li J, Tang Y, Song W, Yu Y, Yang W, et al. Comparative life-cycle assessment of microalgal biodiesel production via various emerging wet scenarios: Energy conversion characteristics and environmental impacts. Energy Convers Manag. 2022;257: 115427.
-
Lim HR, Khoo KS, Chia WY, Chew KW, Ho S-H, Show PL. Smart microalgae farming with internet-of-things for sustainable agriculture. Biotechnol Adv. 2022;57: 107931.
-
Lobine D, Rengasamy KRR, Mahomoodally MF. Functional foods and bioactive ingredients harnessed from the ocean: current status and future perspectives. Crit Rev Food Sci Nutr. 2022;62:5794–823.
-
Salehipour-Bavarsad F, Nematollahi MA, Pistocchi R, Pezzolesi L. Algal food safety: Possible contaminations, challenges of harmonized quality assessments, and suggested recommendations for the nascent industry of microalgae-based products. Algal Res. 2024;81: 103579.
-
Gaurav K, Neeti K, Singh R. Microalgae-based biodiesel production and its challenges and future opportunities: a review. Green Technol Sustain. 2024;2: 100060.
-
Kumar G, Shekh A, Jakhu S, Sharma Y, Kapoor R, Sharma TR. Bioengineering of microalgae: recent advances, perspectives, and regulatory challenges for industrial application. Front Bioeng Biotechnol. 2020;8:914.
-
Behera B, Unpaprom Y, Ramaraj R, Maniam GP, Govindan N, Paramasivan B. Integrated biomolecular and bioprocess engineering strategies for enhancing the lipid yield from microalgae. Renew Sustain Energy Rev. 2021;148: 111270.
-
Ajjawi I, Verruto J, Aqui M, Soriaga LB, Coppersmith J, Kwok K, et al. Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat Biotechnol. 2017;35(7):647.
-
Santin A, Balzano S, Russo MT, Palma Esposito F, Ferrante MI, Blasio M, et al. Microalgae-based PUFAs for food and feed: current applications, future possibilities, and constraints. J Mar Sci Eng. 2022;10:844.
-
Jebali A, Sanchez MR, Hanschen ER, Starkenburg SR, Corcoran AA. Trait drift in microalgae and applications for strain improvement. Biotechnol Adv. 2022;60: 108034.
-
Mou JH, Qin ZH, Yang YF, Liu SF, Yan W, Zheng L, et al. Navigating practical applications of food waste valorisation based on the effects of food waste origins and storage conditions. Chem Eng J. 2023;468:143625.
-
Pleissner D, Lau KY, Ki Lin CS. Utilization of food waste in continuous flow cultures of the heterotrophic microalga Chlorella pyrenoidosa for saturated and unsaturated fatty acids production. J Clean Prod. 2017;142:1417–24.
-
Law AWS, Rubio Rincón F, van de Vossenberg J, Al SZ, Welles L, Rene ER, et al. Volatile fatty acid production from food waste: the effect of retention time and lipid content. Bioresour Technol. 2023;367:128298.
Acknowledgements
Pau Loke Show would like to acknowledge Khalifa University (FSU-2024-001) for support under project reference number 8474000580. One of the authors (Kuan Shiong Khoo) gratefully acknowledges the financial support provided by the National Science and Technology Council, Taiwan (Project number: 112-2222-E-155-005), and the Department of Chemical Engineering and Materials Science, Yuan Ze University, Taiwan, under the New Faculty Research Start-Up Fund Scheme (Project numbers: 303014-1 and 303014-2). The authors would also like to acknowledge the Curtin Malaysia Research Institute (CMRI) for providing matching fund support for Project ID: 6035. Moreover, we would like to acknowledge that the icons used in the figures of this work were sourced from Flaticon (www.flaticon.com), with each icon individually designed by respective creators.
Funding
National Science and Technology Council, 112-2222-E-155-005, Department of Chemical Engineering and Material Science, Yuan Ze University, Taiwan, 303014-1 and 303014-2, Khalifa University (FSU-2024-001), 8474000580 and Curtin Malaysia Research Institute (CMRI) Project ID: 6035.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, G., Chong, J.W.R., Khoo, K.S. et al. Upcycling food waste for microalgae cultivation toward lipid production in a closed-loop and system-integrated circular bioeconomy. Biotechnol. Biofuels Bioprod. 18, 74 (2025). https://doi.org/10.1186/s13068-025-02679-6
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1186/s13068-025-02679-6